
New testing equipment for seal integrity of pharmaceutical packaging improves accuracy and flexibility.

New testing equipment for seal integrity of pharmaceutical packaging improves accuracy and flexibility.

As part of its work to combat the opioid crisis, the agency is considering fixed-quantity, unit-of-use blister packaging for certain immediate-release opioid analgesics.
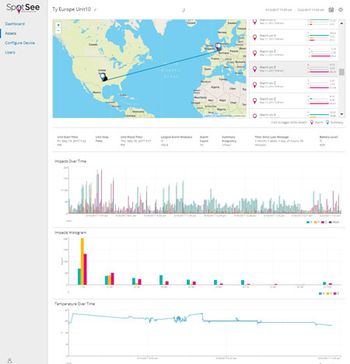
Considerations for monitoring impact, vibration, and temperature during package transportation and storage.

TraceLink will participate in the agency’s Pilot Project Program under the Drug Supply Chain Security Act.
Leachable silicone oil may have an effect on large-molecule APIs, making it important to establish a robust analytical method to detect and quantify the substance.

INTERPHEX 2019 presented solutions for ophthalmic containers, materials, and equipment for forming primary containers, child-resistant and tamper-evident (TE) cartons, and cold-chain packaging.

A UK-based company, Atelerix, has revealed that it has closed a second round of funding worth £700,000 (US$911,000).

Contract packaging and clinical supply service company Sharp invested $21 million to expand its packaging capacity and capabilities in Pennsylvania.

Contract packagers expand operations and services to accommodate growing need.

Legacy Pharmaceuticals has entered into a collaboration with SCHOTT to solve a technical challenge of leaching that is occurring with an antiviral drug.

The company will add a cGMP packaging line and a new filler on its existing packaging line at its Mirabel, Québec, Canada, site.

In a keynote session at INTERPHEX 2019, experts will review and debate the issues and present potential solutions for contamination issues in aseptic manufacturing.

The agency published guidance on how to determine the placement and content of pediatric information in the labeling of drugs and biologics.

The organizers of Pharmapack Europe have revealed their prediction that the drug delivery and healthcare packaging industry will experience significant growth during 2019.

Serialization-ready packing equipment and services to manage data assist companies with DSCSA compliance.

The Dual Cell-E Robotic Palletizer from ESS Technologies integrates a FANUC robot with custom-designed end-of-arm tooling for palletizing in a small footprint.

At INTERPHEX 2019, MG2/MG America stages a packaging/processing summit; exhibitors offer packaging equipment innovations for parenteral products.

The Marchesini Group will introduce the CMP pharma inspection system to the US market at INTERPHEX 2019.

The company is voluntarily recalling four lots of Drospirenone and Ethinyl Estradiol Tablets, USP because of the possibility they may contain defective blister packs and incorrect tablet arrangements.

As demand increases, new materials and machines broaden options for blister packaging.

GS1, a global supply-chain standards organization, launched a new messaging standard in collaboration with GS1 US to help meet the requirements of the US Drug Supply Chain Security Act (DSCSA) for salable returns of serialized prescription drugs.

Nine award winners from the 2019 edition of Pharmapack Europe, covering innovations across drug delivery, packaging, and materials and components, have been announced by the event organizers.

Supply chain players are eligible to participate in FDA’s pilot program to develop an electronic system for tracking drug products.

Honeywell introduces Aclar Accel barrier film with faster production and delivery for pharma packaging.

PCI Pharma Services expanded capacity of its commercial packaging site in Illinois.