
Gerresheimer and Nelson Labs will work together to conduct extractables and leachables testing on upcoming primary packaging solutions.

Gerresheimer and Nelson Labs will work together to conduct extractables and leachables testing on upcoming primary packaging solutions.

Pharma companies set goals and adopt more sustainable alternatives.

Product safety and security are paramount when it comes to container closures.

Rapidly growing industry must overcome supply chain constraints.
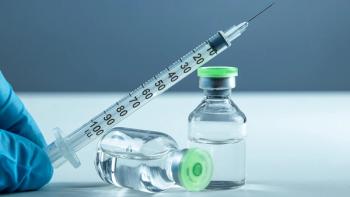
Automated finished product inspection has been widely adopted in the bio/pharmaceutical industry.

Automation of pharmaceutical packaging saves costs and time, say contract packagers.

In this episode, Grant Playter, assistant editor, sits down with Rosalind Beasley, vice president, Digital Innovation, Dot Compliance, to discuss the current state of the compliance and validation industry.

Exhibitors display innovations for parenteral and solid dose products.

In this episode, experts share their insights into important drug packaging advances of the past, present, and future.
Technological advances are progressing pharmaceutical packaging to overcome the challenges of the future.
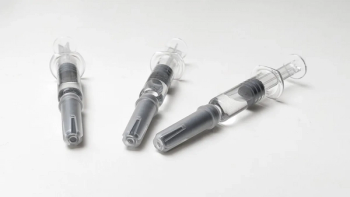
Demand spurs innovations in syringes and syringe fill/finish operations.

Connect in Pharma will host an awards ceremony to showcase innovations in sustainable pharmaceutical packaging.

Yourway intends to construct a new manufacturing facility in the Dublin Airport Logistics Park.

Demand spurs innovations in syringes and syringe fill/finish operations.

The inaugural Connect in Pharma event will take place on 14 and 15 September in Geneva, Switzerland.

The final guidance addresses safety aspects of container and carton labeling design.

Innovations address sustainability, serialization, and supply-chain issues.

Schott has announced the inauguration of its new high-end polymer prefillable syringe manufacturing facility in Mülheim, Germany.

Catalent will invest $350 million into integrated biologics drug substance and drug product manufacturing at Bloomington, Indiana facility.

Tjoapack has doubled its packaging capacity with the completion of its Netherlands facility expansion.

Exhibitors display primary packaging, secondary packaging, and machine innovations at this year’s INTERPHEX.

Innovations address supply-chain constraints, serialization, and new requirements for sustainable and patient-centric designs.

Catalent has announced the completion of a $30 million project at its facility in Limoges, France focused on biopharmaceutical development and drug product manufacturing.

CDMO Almac Pharma Services expanded its European ultra-low temperature packaging capabilities.

Innovations address sustainable and patient-centric designs.