
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2012 edition from Koslow Scientific Company and Vaisala.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2012 edition from Koslow Scientific Company and Vaisala.

Visitors will see many packaging innovations at the annual industry exhibition.

In a world where product recalls can mean the end of a company, all batches must be perfect.

The US Pharmacopeia announced a draft standard containing best practices for ensuring that drugs can be traced to their original manufacturer, are not counterfeited or adulterated, and can be transported to their intended destination without compromising quality.

Packaging innovations boost productivity, meet regulatory requirements, and protect products.
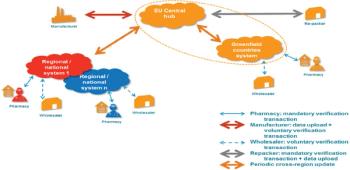
Counterfeit medicines are an increasing threat to the EU supply chain and there is need for a standard identification solution across Europe.

Counterfeiting continues to be a huge challenge affecting both branded and generic products, which, although a worldwide issue, is especially prominent in developing countries.

It is very difficult to measure the problem of counterfeiting accurately from year to year; by definition criminals don?t file tax returns or publish quarterly earnings.

In an age dominated by the internet and uncertainty over the best packaging security methods to employ, counterfeit medicines have the ideal environment to thrive.

Inspection systems play a big part in ensuring product quality.

PTE interviews Leigh Jordan, UK Sales Manager at Cognex UK Ltd, about the importance of code readability when using anticounterfeiting solutions such as 2D datamatrix codes.
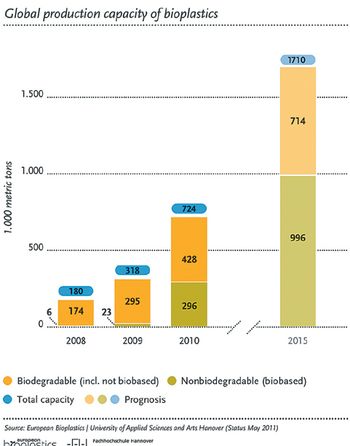
Drugmakers and packagers are pursuing various initiatives to reduce their carbon footprints. This article contains bonus material.

Smartphones could become the product-authentication tool of choice. Contains online bonus material.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2011 edition from Chemineer and IDS.

Many child-safe package designs help improve compliance and provide tamper evidence.

Terry Novak, president of Norwich Pharmaceuticals, on recent industry trends.

The authors share their approach and experience working in complex, multicompany environments for in-licensed products to develop successful chemistry, manufacturing, and controls packages for managing outsourcing partnerships.

The European Medicines Agency has updated the template for package leaflets to make the information easier for patients to understand.
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the May 2011 edition from David Round Company and Globe Medical Tech.

Extending a drug product's shelf life while maintaining its strength, quality and purity is a challenge for pharma companies.

There are a number of points to consider where secondary packaging is concerned.

Legislation has prompted the pharma industry to seek technologies that can be used to secure a product throughout the supply chain.

More challenges face the pharma packaging industry today than ever before, according to Domino Printing Sciences' global life sciences sector manager.

In light of the challenges facing the pharma industry, such as changing patient demands and changing demographics, the relationship between the packaging, the consumer and the product is being completely reconsidered.

Research conducted in the UK by the University of Leeds and Luto Research shows that many commonly-used phrases on medicine labels are easily misunderstood.