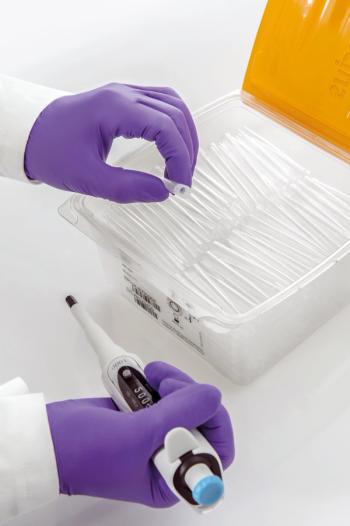
FlexiBulk tip packs save space and effort in the laboratory

FlexiBulk tip packs save space and effort in the laboratory

PTI's next-generation VeriPac Inspection System, the VeriPac 310, uses new vacuum-decay technology to increase leak-detection accuracy for a range of flexible and rigid packages and containers.

Innovations include multimedia packaging and improved filling systems.

Schreiner MediPharm's low-migration labels for plastic containers use qualified adhesive systems, materials, and inks.

3C! Packaging's Clear Code coating enhances laser-etching on pharmaceutical packaging.

Revisions to USP General Chapter may impact evaluation of sterile product package integrity.

Catalent acquires Pharmapak Technologies, a pharmaceutical packaging company based in New South Wales, Australia.

Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.

Drug makers back alternative to FDA labeling update rule.

Implementing serialization on a packaging line requires a complex integration of hardware and software and often requires that new and existing systems work together.

Temperature-controlled packaging trends include prequalified systems that simplify adoption, reusable systems that are more sustainable, and new temperature-monitoring technology.

Recipharm expects to be rolling out further serialization projects during 2015.

UPS expands its special commodities program to more countries.

A new machine is designed for inspection of prefilled syringes.

The company has increased capacity for cold storage and controlled drug substances handling at its European facilities.

Careful selection of a contract packager should include interviews, surveys, and on-site visits.

UPS Temperature True Packaging provides four prequalified packaging system options for temperature-sensitive products with different time and temperature requirements.

The Shantha facility will be Sanofi's second, in addition to its site in Frankfurt, for production of Insuman insulin.

Market changes are driving pharmaceutical companies to consider new ways to mitigate risk in the cold chain.
Better barrier materials and software modeling streamline the blister-package design process.

Recipharm has completed a serialization project for China on behalf of one of its customers.

Packaging film suppliers offer new materials and alternatives to Barex copolymers.

Rommelag's all-electric aseptic blow-fill-seal packaging system encapsulates the filling point and can be installed in a cleanroom.

A horizontal flow wrapper separates vials and individually packs them at high speed using a long-dwell sealing system with ultrasonic or heat-sealing technology.

Comar's 18-mm SecureCap child-resistant closure for eye drops and nasal sprays meets US Consumer Product Safety Commission for products containing 0.08 milligrams or more of imidazolines.