
Noting traditional clinical trials for COVID-19 convalescent plasma will take time, FDA is allowing physicians to submit requests for single-patient emergency INDs.

Noting traditional clinical trials for COVID-19 convalescent plasma will take time, FDA is allowing physicians to submit requests for single-patient emergency INDs.

FDA is offering advice and added flexibility to help sponsors adjust ongoing and planned clinical research programs during the COVID-19 outbreak.

USP technical advisors will offer assistance to drug developers to ensure material quality and testing.

The Pharmacovigilance Risk Assessment Committee (PRAC) of EMA has recommended that women stop taking 5-mg ulipristal acetate (Esmya and generic medicines) for the treatment of uterine fibroids while a safety review into potential liver injury risk is performed.

FDA postpones routine domestic facility inspections due to the COVID-19 pandemic.
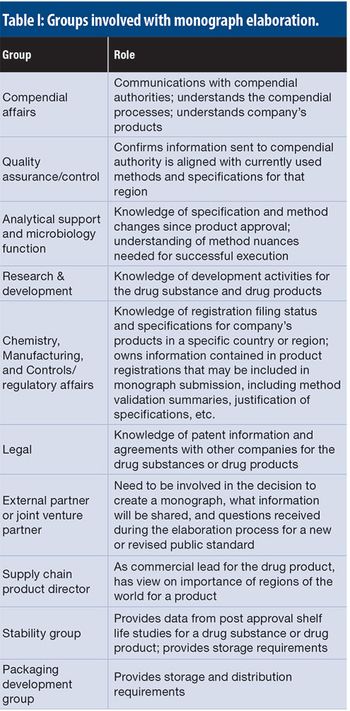
This article details the more operational aspects of monograph submissions, answering the question of how to participate.

The authors present a case study with raw materials and excipients, where a consistent, cross-functional approach is needed to ensure the appropriate selection, sourcing, testing, and filing of the materials used to manufacture bio/pharmaceutical products in a global environment, ensuring compliance with applicable compendial and regulatory requirements.

This article summarizes all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world. This look into what is on the horizon is important to help companies prepare for the inevitable changes and ensure the continued supply of quality medicines to patients globally.

This series is intended to address the challenges for the industry to comply with pharmacopoeial requirements. This article returns to this important topic with a case study at the intersection of monograph development and compliance.

Find links to pertinent regulatory and standard setting resources, guidance documents, and guidelines.
Connect with pharmaceutical and healthcare regulatory authorities around the world via this directory.

With some FDA inspections on hold, will the US drug supply maintain its quality standards?

Monographs are developed based on the submission of information and materials from a company having regulatory approval for the product, and this submission feeds into the pharmacopoeia revision process.


This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

This article returns to the topic of complying with pharmacopoeial requirements with a case study at the intersection of monograph development and compliance.

In January 2020, the agency finalized six clinical development and manufacturing guidance documents and drafted new guidance on what would qualify new gene therapies as orphan drugs.

Data supporting the quality and safety of product must meet the ALCOA+ elements in order to avoid regulatory citations for data integrity issues.

This case study is based on the experience of one of the authors but is applicable to all companies across the broader industry, illustrating the potentially surprising point that some compliance difficulties may be of the company’s own making.

This article details the more operational aspects of monograph submissions, answering the question of how to participate.

This article explores a proactive advocacy approach that a bio/pharmaceutical company may take through participation in the development of new and revised monographs in the various pharmacopoeias.

This article presents a case study at the intersection of monograph development and compliance.

This final article in the series has two purposes: to summarize all the considerations that go into a company’s compendial affairs program and to look ahead at topics that will likely result in further evolution in the pharmacopoeias around the world.

EMA, along with its partners across the European medicines regulatory network, are keeping a close watch on the pharmaceutical supply chains in the European Union as a result of the potential impact of COVID-19.

The agency is postponing the inspection of most foreign facilities through April 2020.