
The company recalled a variety of products due to the potential of microbial contamination.

The company recalled a variety of products due to the potential of microbial contamination.

Congressional measures lack the support to move forward.

A consent decree of permanent injunction was entered between the United States and the two companies due to unapproved, mislabeled, and adulterated drugs.

The company has extended its voluntarily recall of one lot of paliperidone extended-release tablets to the consumer level due to dissolution test failure.

The agency sent a warning letter to A-S Medication Solutions LLC after it found the company didn’t comply with drug listing file requirements.

FDA Commissioner Scott Gottlieb announced the July meeting as part of his commitment to address opioid abuse.

FDA sent a warning letter to drug compounder DCA, Inc. dba Beacon Prescriptions for failing to ensure sanitary conditions.

The company is voluntarily recalling one lot of Eliquis after a patient discovered that a bottle labeled as Eliquis 5 mg was found to contain Eliquis 2.5 mg tablets.

The three regulatory agencies have agreed to data requirements for development of new antibiotics.

In its June meeting, the agency’s Pharmacovigilance Risk Assessment Committee discussed medicine safety reviews.

FDA asked Endo Pharmaceuticals to remove Opana ER from the market, citing the potential for abuse.

Yusef Manufacturing Laboratories, LLC received an FDA warning letter citing CGMP violations at its Clearfield, UT facility.

The author shares his opinion on the challenges presented by the Internet of Things and what companies need to consider when choosing suitable architectures to manage serialization data.
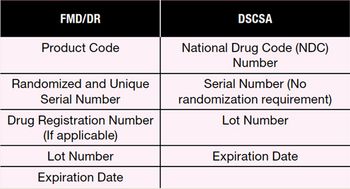
The author discusses upcoming serialization and transaction data collection regulations including the US Drug Supply Chain Security Act, the European Union Falsified Medicines Directive, and the EU Delegated Regulation.

Serialization and complementary authentication technologies are needed in order to meet DSCSA and FMD regulations.

The impact of Brexit on the European drug approval regulatory framework presents challenges for EMA.

The company recalled the tablets due to a packaging error.

The agency published an action plan to nurture innovation and drug development by SMEs.

The company voluntarily recalled one lot of Brilinta 90 mg professional samples because it contained another medicine.

The agency released guidance for industry regarding the United Kingdom’s withdrawal from the European Union.

Approval of breakthrough therapies requires expedited quality assessment.

The authors summarize the current regulatory expectations regarding the number of PPQ batches required and provide potential approaches that can be used to determine and justify the number of PPQ batches.

Informing your clients of possible changes in equipment is imperative when upgrading a laboratory, says Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

The company was cited for cGMP violations at its Irvine, California facility.

The agency met with the representatives of the East African Community to discuss the creation of a networking agency.