
The agency cited Morton Grove Pharmaceuticals for inadequate quality control procedures.

The agency cited Morton Grove Pharmaceuticals for inadequate quality control procedures.

The agency sent a warning letter to Chongqing Pharma Research Institute Co., Ltd. citing data integrity violations.

Dr. Janet Woodcock said implementation of Informatics Process Management is a priority during the latest Director’s Corner podcast.
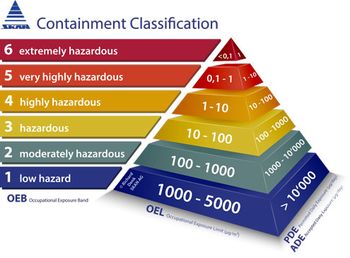
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Policies limiting imports and immigration generate uncertainty for US and foreign firms

Siegfried Schmitt, PhD, Principal Consultant at PAREXEL, discusses how to mitigate risk in a global regulatory environment.

A survey on risk-based predictive stability tools reveals how pharma companies are leveraging advanced stability approaches throughout the drug development process.

A robust quality agreement and good communication scheme can help avoid and alleviate regulatory concerns.
The DME Facility Focus survey revealed best practices for coping with the challenges of aging facilities and implementing facility modernization.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

Pharmaceutical Technology asked Siegfried Schmitt, principal consultant at PAREXEL, about the importance of quality agreements in the sponsor/contractor relationship.

As the November 2017 deadline nears, a surprising number of companies still don’t have a serialization plan in place. New programs aim to get them compliant in time.

Efforts to harmonize the fragmented regulatory framework for pharmaceuticals in the European Union continue to be a challenge.

President Trump calls for faster FDA approvals and lower drug prices.

This article reviews experiences with the outcome of in-house audits, audits by third parties, and purchased audit reports.

Having an effective and granular data management process in place will enable companies to meet the requirements of IDMP as well as help usher in a new age of digital-based identification, in which organizations can easily share data across borders.

The agency cited the company’s Kansas facility with CGMP violations similar to problems found at other Hospira facilities.

FDA plans to initiate its quality metrics program as industry continues to push back.

Avella issued a nationwide recall of sterile products produced at the Advanced Pharma Houston location due to inaccurate labeling.

The company is voluntarily recalling one lot of Edex due to a lack of container closure integrity.

The agency’s CHMP recommended six drugs for market approval, including one orphan drug, during its February meeting.

The agency’s CHMP recommended conditional marketing authorization for the hormone replacement therapy for the treatment of chronic hypoparathyroidism.

In 2016, FDA approved 630 ANDAs and tentatively approved 183 ANDAs, the highest number to date, according to the report.

The authors discuss regulatory and patent issues with combination products.

The district court ordered Pick and Pay Inc./Cili Minerals to cease operations after it unlawfully manufactured and distributed unapproved new drugs, misbranded drugs, adulterated dietary supplements, and misbranded dietary supplements.