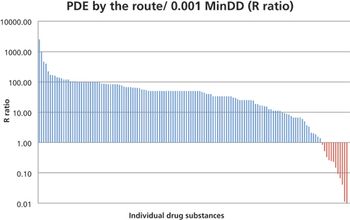
In this study, the authors investigated the relationship between the 0.001 MinDD and the PDE values for 140 drug substances as an attempt to identify high-risk groups of products for patient safety. This comparison can serve as a method for prioritization of APIs for development of PDEs.