
Generic versions of AstraZeneca’s blockbuster Crestor will hit the US market after a federal judge refused to issue a restraining order blocking the approval of rosuvastatin ANDAs.

Generic versions of AstraZeneca’s blockbuster Crestor will hit the US market after a federal judge refused to issue a restraining order blocking the approval of rosuvastatin ANDAs.

The committee voted unanimously in favor of approving the drug, but the majority supported implementing additional risk management.

FDA issued a warning letter to the Worthing, UK facility for cross contamination and microbial contamination cGMP violations.

The agency says the increasing requests for orphan drug designation has resulted in a change in FDA’s review goals.

The agency reviews hemophilia A, skin, and diabetes treatments, among others.

The agency completes its risk assessment of the blood cancer treatments.

The agency announced that it has completed the review of the GDUFA backlog one year ahead of schedule.

The agency says that the routine large-scale compounding of drugs that are exact copies of existing medications undermines the the drug approval process.

The draft guidance addresses control of elemental impurities in harmonization with implementation of ICH Q3D guideline.

FDA cited Guangzhou Haishi Biological Technology Co., Ltd. with CGMP violations.

Agency guidance and industry standards aim to reduce lapses and improve quality operations.
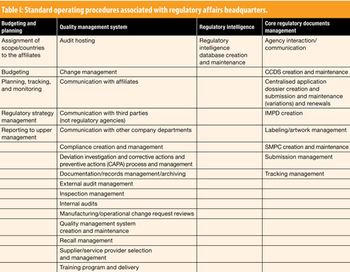
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.

Designing systems using the principles of good documentation practice, including validated audit trails, is a key piece of a manufacturing data integrity program.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

China and India are also increasing inspections and becoming more exigent about data integrity and cGMPs.

Data integrity and cGMP issues demand closer scrutiny of suppliers. Bribery and corruption may become the next supply chain flashpoint.

The impact of pharmaceutical manufacturing on the environment has triggered demands for tighter environmental controls in EU and national legislations.

ICH detailed the highlights of the council’s June 2016 meeting.

The agency is following up on a February 2016 inspection of the facility that found GMP violations.

The agency recommends Zalmoxis, a new cell-based therapy to support stem cell transplantation in patients with high-risk blood cancer.

The agency has suspended recommendation of Riluzole Alkem due to flawed study results.

The agency publishes draft reference material for implementation of FDA’s quality metrics guidance.

China’s State Council issued a notice authorizing a trial plan for a new drug marketing authorization holder system for 10 provinces.

OPKO’s newly approved extended-release capsule uses Catalent’s OptiShell softgel capsule technology.

The Pharmacopoeial Discussion Group approved monographs and plans to harmonize several others.