
The agency provides quality, development, manufacturing, and labeling recommendations.

The agency provides quality, development, manufacturing, and labeling recommendations.
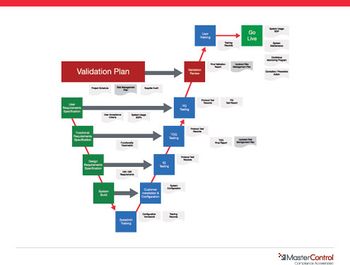
Computerized systems can solve some of the data integrity problems with conventional paper-based systems.

FDA sent a warning letter to The Compounding Pharmacy of America for deficiencies in sterile manufacturing.

The agency published guidance on the nonclinical evaluation of osteoporosis treatments.

FDA approved Vaxchora intended for travelers who are at risk for the disease.

Mandatory use of the periodic safety update report repository becomes mandatory on June 13.

Sharing un-redacted inspection reports between FDA and EMA may reduce duplicate inspections of facilities.

The agency publishes three final guidance documents on drug compounding.

The agency reiterated its earlier decision to require suffixes in biosimilar naming, but was unclear on suffix meaning.

The agency published a report on fostering the development of advanced therapy medicinal products.

FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.

Investment in talent and infrastructure, more quality-by-design skills, and increased communication with global regulators will be needed to combat compliance issues at API and drug manufacturing facilities in India.

FDA approved dalizumab a monoclonal antibody for the treatment of multiple sclerosis.

During its May 2016 meeting the CHMP recommended the approval of six medications in the EU, including three generic drugs, treatments for hepatitis C, and Type 2 diabetes.

FDA issues a warning letter to Megafine Pharma Limited for data integrity violations.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on data integrity.

More efforts are needed to raise awareness of biosimilars among physicians and patients in Europe and address scepticisms about the quality and safety of biosimilars.

Pharma has voiced strong opposition against Britain’s departure from the EU.

FDA approved Probuphine, the first buprenorphine implant approved in the United States for the treatment of opioid dependence.

The agency publishes draft guidance on assessing the adhesion of transdermal delivery systems and topical patches.

Samsung Bioepis and partner Biogen announced on May 30 that the European Commission approved Flixabi for the treatment of six inflammatory conditions.

Sandoz is seeking approval for the same indications as Roche’s reference product MabThera.


FDA accepted for review Samsung Bioepis’ BLA for SB2, a biosimilar to Remicade (infliximab).