Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.
Agnes Shanley is senior editor of Pharmaceutical Technology.

Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.
Air transport continues to be the most secure way to ship valuable therapies, but it is also the riskiest. Standards are helping to improve service quality.

When it comes to dissolution testing, research is focusing on ways to extend its reach, improve in-vitro and in-vivo correlation, and make real-time release testing a reality.

Traditional barriers between upstream and downstream bioprocessing are slowly starting to break down, as biopharma embraces more advanced analytics and process control.

Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.

Demand for direct-to-patient services is exploding. Michael Sweeney, senior director of patient-centric logistics at World Courier, discusses issues that the new model presents.

Dissolution testing remains one of the pharmaceutical industry’s most straightforward, least expensive QC tools. Research is focusing on ways to extend its reach, improve in-vitro and in-vivo correlation, and make real-time release testing a reality.

As more continuous processes move toward commercialization, users and equipment vendors are working to speed changeovers and address other needs.

ASTM’s E-55.05 committee aims to turn best practices into industry standards in order to eliminate variability and allow a 70-year-old process to leverage 21st century tools, technologies, and practices.

Shipping biopharmaceuticals in single-use containers requires a thorough understanding of the distribution cycle and potential transportation risks.

Most pharma companies still aren’t ready for serialization, and only a few have coordinated traceability efforts with their CMOs, according to Tracelink’s latest readiness report.

Taking vendor product data at face value puts pharmaceutical manufacturers and their supply chain partners at risk.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport with Pharmaceutical Technology.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

Can an Irish analytics company and its CEO bring pharma closer to 21st-century practice?

While more companies are embracing taggants, researchers are developing new technologies that will be extremely difficult to reproduce.

Chemical engineers and chemists each bring unique skills to API process development and optimization. Success depends on their close collaboration, at both sponsor and CDMO facilities.

CRLs put the brakes on drug development and damage corporate reputation and stock prices. Upfront investment and better sponsor oversight are ways to prevent them.

Research suggests that working with a single contract partner can reduce development time and improve economics.

Joe DiMasi, director of economic analysis and research associate professor, Tufts Center for the Study of Drug Development (CSDD), discussed research into the impacts of working with single source contractors with Pharmaceutical Technology.

Pharmaceutical companies and contract manufacturing organizations report lack of readiness for the US Drug Supply Chain Security Act serialization deadline.

A review of 2017 advancements from the Open-SCS working group with Charlie Gifford, group technical director.

Everyone may not be ready for the deadline, but open standards based on GAMP and GS1 will soon be released; more companies are also leveraging what they’ve learned from serialization to improve overall efficiency.
Despite the challenges and high cost of development, vaccine innovation is at an all-time high, as new approaches aim to improve global access.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.

Most pharma supply chain partners believe that manufacturers should pay for blockchain implementations, and own the data.

Just as FDA strengthens ties between reviewers and plant inspectors, proactive manufacturers are involving different disciplines in preparation for FDA audits.
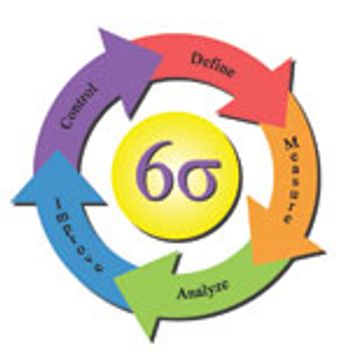
Instead of rigidly applying statistical tools, experts suggest that pharma embrace statistical thinking, but focus on reducing variability and adding value for patients.

Lawrence Yu, deputy director of the Office of Pharmaceutical Quality (OPQ), Center for Drug Evaluation and Research (CDER), and his FDA colleague, Michael Kopcha highlight the importance of Six Sigma approaches to continuous improvement in the pharmaceutical industry.

Internet of things, advanced analytics, and blockchain solutions such as smart contracts promise to give manufacturers more control over products and supply chains.