
A recent Ernst & Young survey highlights the challenges facing Indian pharma
Agnes Shanley is senior editor of Pharmaceutical Technology.

A recent Ernst & Young survey highlights the challenges facing Indian pharma

As public criticism of drug prices intensifies, and healthcare moves to "value-based" pricing and purchasing, Sloan-Kettering’s interactive, online tool Abacus offers insights into the factors that must go into pricing a drug

Despite the buzz about biologic-based drugs, small molecule-based drugs are still the mainstay treatment.

Aging facilities and inadequate quality systems create drug shortages. Preventing them will require harmonized regulations, and management support for continuous improvement and innovation.


As a special session at Interphex 2015 this week made clear, few pharma companies are ready for serialization and aggregation deadlines. The disconnect between pharmaceutical manufacturers and their contract partners poses a special risk.

Statistics presented in a March webinar suggest a strong, but highly fragmented, small-molecule API market

Ionic liquid technologies offer a new way to improve bioavailability.

QbD represents a breakthrough in thinking, but does it go far enough to address today's business challenges? NeoStem has expanded it to include business and market issues, in "Development by Design." Could this be (or is it becoming) a new model for pharma?
Whether outsourcing or developing cell therapies in-house, success demands a focus on quality, cost of goods, and sustainability from the start.

CMO executives share their opinions on where outsourcing is going and what is driving market change.

As CMOs shed their old toll processing role, sponsors can expect the right questions, proactive communication, and a firm grasp of risk management and tech transfer from contract service providers.
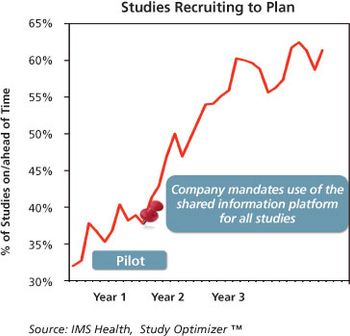
Data sharing and a common data model can improve CRO partnerships and trial results.
After launching a new mammalian cell platform, FUJIFILM Diosynth Biotechnologies U.S.A., has acquired fast-track vaccine manufacturing knowhow and a major presence in Texas’ emerging biocorridor with Kalon, its first acquisition.

Better equipment and automated processes will be key. So, too, will the right way of approaching outsourcing relationships.

Sandoz announces their version of filgrastim, a follow-on biologic for the treatment of neutropenia, is as safe and effective as Amgen's Neupogen.

A Bain research study on shareholder value draws conclusions about pharma leaders.

An index from the Access to Medicine Foundation ranks GSK as most effective in making products accessible.

Reports of widespread cGMP noncompliance at various Indian pharmaceutical companies raise awareness on the need for one quality standard for both developing nations and those with stricter regulations.

Tufts research suggests costs to launch a new drug have doubled since 2003, but some question conclusions.