Pharmaceutical Technology Editors
Articles by Pharmaceutical Technology Editors

The US Food and Drug Administration announced that it had filed a Consent Decree on Dec. 23, 2008 and was awaiting the court?s entry of a permanent injunction that bars Actavis (Hafnarfjordur, Iceland), its officers Sigurdur Oli Olafsson and Douglas Boothe, and its subsidiary Actavis Totowa (Totowa, NJ) from manufacturing and distributing drugs at the Actavis Totowa facilities.

Also, Bristol-Myers Squibb forms collaboration with ZymoGenetics, Hana Biosciences appoints VP of regulatory affairs, more...

Also, Novozymes Biologicals settles pollution case with the US Department of Justice; EntreMed restructures management team; more...

Michigan State University researchers have discovered a technique for viewing whole cells to gain an understanding of protein inclusion bodies.

A California jury ordered Pfizer (New York) to pay $38 million to the Ischemia Research and Education Foundation (IREF), a medical-research nonprofit group, for stealing trade secrets to develop its "Bextra" analgesic.

A book explains the many pharmaceutical applications of polymers from natural sources.
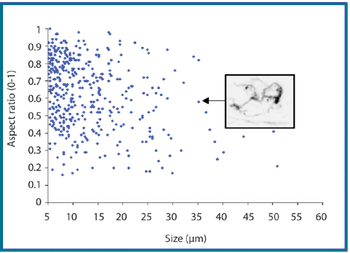
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.

Also, Crucell and DSM announce deals with GSK, Talecris, and CSL; Nobel Prize winner Luc Montagnier joins Viral Genetics; more...

The US Food and Drug Administration dedicated historic Building One at the White Oak Federal Research Center in Silver Spring, Maryland.

The Pharmaceutical Research and Manufacturers of America (PhRMA) adopted measures to strengthen its Guiding Principles for Direct to Consumer (DTC) Advertisements about Prescription Medicines.

Also, BASF opens lab in Mumbai; Evotek president and CEO to resign; more...

The US Food and Drug Administration released a draft guidance document that contains questions and answers relating to new labeling requirements for over-the-counter drug (OTC) products that are marketed without an approved application under section 502(x) of the Dietary Supplement and Nonprescription Drug Consumer Protection Act of 2006.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2008 edition from Spiroflow Systems and Sepha.

A surprising amount of the discovery and development life cycle is still based on manual and disconnected process steps.

Packaging a drug in a prefilled syringe may help extend a product's patent and distinguish it from competing products.

Also, NicOx and DSM make manufacture and supply pact for naproxcinod drug substance; NovaRx appointed Norrie Russell president and COO; more...

The US Food and Drug Administration has released Guidance for Industry: Cooperative Manufacturing Arrangements for Licensed Biologics, which finalizes the draft from August 1999.

A Centers for Disease Control and Prevention (CDC) study confirmed that severe adverse reactions reported in the fall and winter of 2007 resulted from heparin contaminated with oversulfated chondroitin sulfate (OSCS).

Industry experts are predicting that last week?s terror attacks in Mumbai, India may have at minimum some short-term effects on the pharmaceutical industry outsourcing business in that region.

FDA's Final Rule titled "Amendments to the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals" takes effect Monday, Dec. 8, 2008.

Also, AstraZeneca announces changes to its supply chain operations; Christian Velmer appointed head of Wyeth Canada; More...

The European Commission (EC) published a preliminary report stating that competition in the pharmaceutical industry does not work as well as it should.

Also, Johnson & Johnson will acquire Omrix Biopharmaceuticals for $438 million; Charles River Laboratories promoted Foster Jordan to corporate senior VP of endotoxin and microbial detection products; more...

During its roundtable discussion titled "Follow-On Biologic (FOB) Drugs: Framework for Competition and Continued Innovation," The Federal Trade Commission (FTC) discussed likely effects, patent issues, and regulatory exclusivity periods concerning FOBs.

Ranbaxy Laboratories (Gurgaon, Haryana, India) responded to an investigation of violations at two of its manufacturing plants, according to a Reuters Health report.

Although the number of annual new-drug approvals in the United States has steadily declined during the past ten years, pharmaceutical companies still prefer to introduce new products in the US first, according to a report published by the Tufts Center for the Study of Drug Development.

Also, BASi opens European office in Warwickshire, UK; Acusphere's Howard Bernstein Resigns; More...

US marshals seized 11 lots of heparin from Celsus Laboratories (Cincinnati, OH) at the request of the US Food and Drug Administration.

Also, Patheon opens new European headquarters, Cynvec appoints Frank D. Stonebanks president and CEO, more...

PAT may reduce costs by helping companies control process variability, improve yields, reduce waste, and produce high-quality therapies consistently. Companies that have not yet embraced PAT may find its potential to reduce expenses a compelling argument in its favor during this time of financial difficulty.



