
3M Drug Delivery Systems Division Vice President Jim Vaughan discusses facility locations and the drug-product inhalation market.

3M Drug Delivery Systems Division Vice President Jim Vaughan discusses facility locations and the drug-product inhalation market.
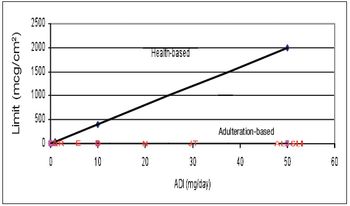
The author discusses how the use of a visible residue limit has made the 10-ppm cleaning limit obsolete in many applications.
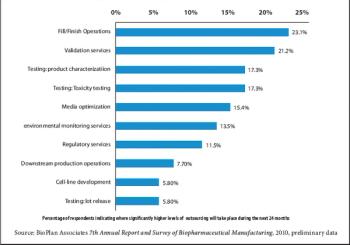
Strategic rather than tactical considerations are driving biopharmaceutical outsourcing.

Sharing too much-or too little-information can have disastrous onsequences.

As contract manufacturers and drug companies meet at Informex, the stage is set for the latest in pharmaceutical chemical development.

FDA impersonators and counterfeit drugs threaten the public's trust in online pharmacies.
Editors' Picks of Pharmaceutical Science & Technology Innovations

A look at the formulation challenges in pancreatic enzyme products.

A recent book provides information about formulating biopharmaceuticals that is easy to swallow.

Vaccine R&D is surging, but continues to raise manufacturing and regulatory challenges.

Regulators and industry move to require inspections of API manufacturing facilities.

When it comes to healthcare rform, we must not overlook investment in innovative technologies.
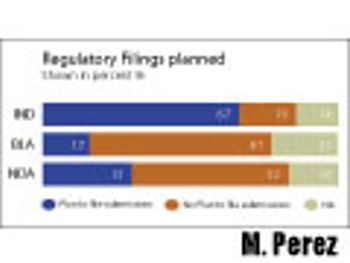
How to cut time and cost by re-using already submitted documents.
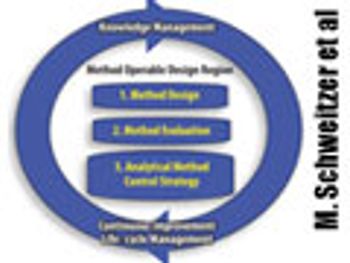
The authors present two concepts to improve robustness and facilitate continuous improvement in analytical methods. This article contain bonus online material.

Leading experts share insight on the current and future direction of process analytical technology. This article contains bonus online material.
We never thought implementing complex changes could become more cumbersome.

The death of a pioneer in molecular genetics recalls age-old questions about social fitness to ensure the ethical uses of scientific advances.