
FDA urges manufacturers to examine how shape, size, and color may affect patient safety and enhance treatment adherence.

FDA urges manufacturers to examine how shape, size, and color may affect patient safety and enhance treatment adherence.

Last year, the Turkish Government unveiled plans to position the country in the top 10 most-preferred locations for R&D investment in the pharmaceutical sector by 2023. This article provides an overview of the incentives introduced under the Technopolis Law and the R&D Act.

High technology assessments are having an impact on biosimilars development in Europe.

PDA works with FDA to create pharmaceutical quality metrics.

CMOs may find opportunities in alternative expression services.
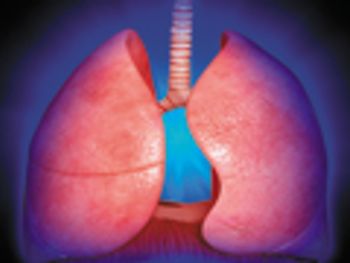
Experts discuss factors affecting drug delivery to the lungs and key considerations when developing inhalation formulations.

ISPE and PDA take on the challenge of recommending quality metrics.

Advances in technology are improving the sensitivity and accuracy of mass spectrometry, increasing its use for the analysis of extractables and leachables.
Content Packages Accelerate MBR Creation

Advanced triple quadrupole technology for improved performance and ease of use.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how human error can be mitigated in pharmaceutical manufacturing.

The biopharmaceutical industry contemplates product innovation within the changing landscape of healthcare.
Automated sample handling, advanced glycan analysis, and specially designed columns are help speed up confirmation of the biosimilarity.
FactoryTalk Software Increases Flexibility
The authors describe the development of a stability-indicating reverse-phase high-performance liquid chromatographic (RP?HPLC) method for the quantitative determination of potential genotoxic impurities present in pemetrexed disodium (form-IV). The chromatographic separation was achieved on a RP?HPLC column (Zorbax SB-Phenyl, Agilent) at ambient temperature with gradient elution using mobile phase A (trifluoroacetic acid [TFA] in water) and mobile phase B (TFA in acetonitrile).

Simple and affordable mass detection for the chromatographer.

Work-in-Process Tracking Software

Software Expands Plant-Specific Analysis
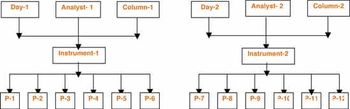
Non-compliance issues show that users find dealing with computer systems challenging.

Although software and the overall warehouse management solution (WMS) are crucial tools, common problems include failure to put in place an efficient warehouse layout best suited to the products being handled, lack of knowledge of the inventory on hand, and inadequate preparation and training of personnel.

Recent regulatory initiatives designed to secure the global pharmaceutical supply chain will directly impact the global supply chain and API manufacturers.

Click the title above to open the Pharmaceutical Technology February 2014 issue in an interactive PDF format.