
Hiden Analytical’s Catlab PCS is a microreactor combined with a mass spectrometer system to characterize and evaluate catalysts while completing general thermal studies.

Hiden Analytical’s Catlab PCS is a microreactor combined with a mass spectrometer system to characterize and evaluate catalysts while completing general thermal studies.

Ross Bow Tie Dispersers, or High Viscosity Dispersers, are heavy-duty mixers designed for heavy pastes and viscous liquids.

Radio Frequency’s Macrowave Dryers feature volumetric heating, moisture reduction, shortened dwell times, and keep temperature uniform throughout temperature-sensitive materials.

FANUC’s M-1iA delta robot is designed for bottling lines with the capability of operating at 120 cycles per second.

Tooling can be damaged by poor handling or problems in process design or material choice.
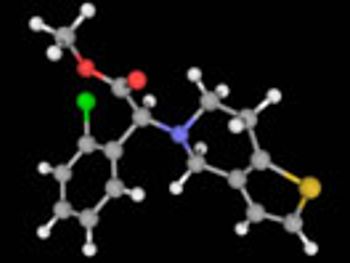
Resolution technologies remain crucial for commercial-scale chiral API production.

Biosimilars, supply chain security, quality metrics, and elemental impurities headline guidance topics on FDA’s 2015 agenda.

Initiatives to speed drug development must pass Congress and special interest groups.
Ligand-binding assays are fundamental to characterizing biosimilars.

EMA is under pressure to exert even tighter standards on biosimilars being marketed in Europe.

Manufacturers face new rules for tracing drugs through the supply chain and compounders face stricter standards.

Market forces may limit the success of CMOs.


Changes in the country’s political landscape may affect the pharmaceutical industry market in the future.

The authors discuss a novel particle engineering technology based on mechano-chemical activation.
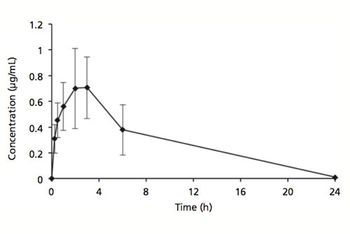
Formulating an injectable solution containing both hydrophilic and hydrophobic drugs is a challenge.
Working with biological matrices and understanding the intended use are crucial.

This article gives an overview of the concept and contents of the revised guidance and outlines how it has changed from the previous version.

Market changes are driving pharmaceutical companies to consider new ways to mitigate risk in the cold chain.

New guidelines focused on the materials of construction in biologic therapy packaging will help vendors prepare comprehensive extractable and leachable testing strategies.
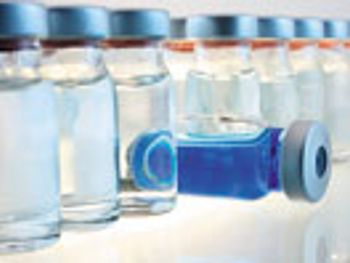
New guidelines and best practices may lead to improved quality and reduced recalls due to visual defects.

Robin Hooker the Director of Healthcare Sector Marketing at UPS discusses issues with maintaining the cold chain in developing markets.

Click the title above to open the Pharmaceutical Technology February 2015 issue in an interactive PDF format.