
Laboratory tests can determine critical cleaning parameters for passivation treatments used to prevent rouge on GMP stainless-steel equipment.

Laboratory tests can determine critical cleaning parameters for passivation treatments used to prevent rouge on GMP stainless-steel equipment.

Pump systems must be designed to meet the needs of specific processes, including preventing cross-contamination and damage due to shear forces.

Early-access schemes aim to make medicines available to patients faster but the regulatory framework remains unclear especially for biologics that involve complex manufacturing.

Drug manufacturers need to work closely with excipient suppliers to ensure supply chain safety.

Reducing regulatory roadblocks requires more than the stroke of a pen.

Although limitations must be overcome, mass spectrometry is having a great impact on biologic development and manufacturing.

Extraction studies demonstrate approaches for evaluating single-use bio-pharmaceutical manufacturing materials.

The authors survey deficiency letters issued for 190 drug master files supporting abbreviated new drug application submissions.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses how to ensure sterility when manufacturing small-scale parenteral batches.

The outlook for the CMO and CDMO industry may be affected by ever-changing politics.

User fee reauthorization is crucial to implementing the Cures Act and refining the approval process.

Longer packouts are becoming the rule, as logistics service providers and sponsors gain experience planning logistics for clinical trials involving novel therapies.

An example of a new cold chain temperature-controlled shipping technology is Cocoon, which was commercialized in November 2016 and designed for use with pallet-sized shippers.
More agile techniques are improving the development of multiparticulate drug-delivery systems.
This article introduces the concepts of pooled variance and the central limit theorem, which are intended for establishing acceptance criteria for blend uniformity data of granular powder blends when a significant degree of sampling bias is involved.

The FlowCam 8100 particle imaging and analysis system from Fluid Imaging Technologies features a camera that can capture images at up to 120 frames per second and high-resolution digital images of individual particles and microorganisms measured in real-time based on their actual size and shape.

The 10-gallon Double Planetary Mixer and Discharge System from Ross, Charles & Son has heavy-duty helical mixing blades, designed for high-viscosity pastes, gels, and putty-like materials.

The firmware version 2.20 for the Excellence Moisture Analyzers HX204 and HS153 from Mettler Toledo includes enhanced user-rights assignment capabilities.
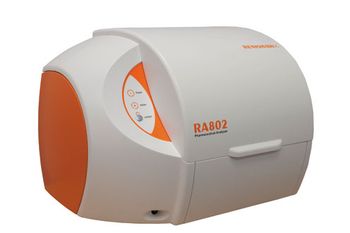
The RA802 Pharmaceutical Analyzer from Renishaw is a compact benchtop Raman imaging system that enables users to formulate tablets by speeding up the analysis of tablet composition and structure.

Click the title above to open the Pharmaceutical Technology February 2017 issue in an interactive PDF format.