
Changes in company ownership shake up the CMO industry.

Changes in company ownership shake up the CMO industry.

Despite the progress made in nanomedicine development, there are several issues that remain unresolved. Raj Bawa, PhD, provides insight.
Quality-by-design principles enhance a thorough understanding of both product and process technology, which is needed for optimization of solid-dosage manufacturing, including processes for improving solubility, such as hot-melt extrusion, softgels, and liquid-filled capsules.

Manufacturers are taking measures to comply with new package safety rules.

The extended-release performance of drug-loaded pellets manufactured by two methods, drug layering and direct pelletization, was compared.

7900 ICP-MS Increases Efficiency

China's regulatory and compliance environment is set to change as the government declares a crackdown on bribery scandals.

Bioprocessors should understand the key factors associated with implementing single-use components or platforms, which include materials of construction, components, system design, and vendor support.
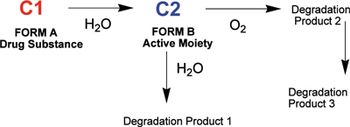
In this article, the authors demonstrate that a normal-phase chromatographic method was stability-indicating for a water-sensitive prodrug. The stress conditions using aqueous and non-aqueous conditions were also compared to understand the information obtained with each approach.

Accelerated testing and production create challenges in documenting product quality.

Using best practices for manual or automatic inspection can improve the inspection process.

Conference sessions from PDA and PharmTech expand educational opportunities at INTERPHEX 2014.

Nanomedicines can offer promising benefits in the diagnosis and treatment of diseases but there are still concerns about the safety and quality of these therapeutics partly because of the lack of clear regulatory guidance. Scott McNeil, PhD, director of the Nanotechnology Characterization Laboratory (NCL), spoke with Pharmaceutical Technology about nanomedicine characterization.

Advances in nanomedicine have provided several potential candidates for safe and effective delivery of siRNA.

Compact Mass Spectrometer Improves Analysis

With nanomedicines on the rise, a new class of non-biological complex drugs (NBCDs), which include nanosimilars, has emerged. As drug regulators are faced with the challenge of defining a framework to ensure the safe introduction of the follow-on nano-therapeutics, Stefan Muhlebach explains why NBCDs cannot be assessed using the standard generic or biosimilar approaches.

ACQUITY QDa Detector Increases Productivity
A survey on postapproval CMC changes was conducted to better understand reporting categories, nature and risks, and review outcomes.

The root cause of drug shortages is mismanagement of variation.
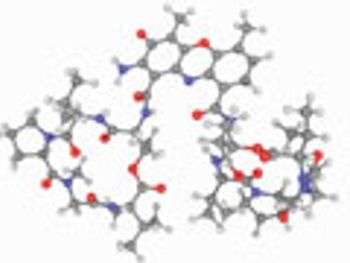
USP evaluates quality attributes for synthetic peptides.
The commercial availability of an increasing diversity of enzymes has led to the growing use of biocatalysts for API synthesis.

SALD-7500nano Expands Measurement of Nanoparticles

Trends driving pharmaceutical packaging include product protection, productivity boosters, and patient adherence improvement.

Click the title above to open the Pharmaceutical Technology March 2014 issue in an interactive PDF format.