
Scientists and industry experts seek effective preventive therapies to combat global disease.

Scientists and industry experts seek effective preventive therapies to combat global disease.

Proposals to make the decentralized procedure more efficient were discussed at the January 2015 EGA conference.

Kason’s K-Series screen replacement program for round vibratory screeners decreases downtime and cost and offers screens designed for any make or model of screener.

GEA Niro Soavi’s PandaPLUS 2000 is a tabletop laboratory homogenizer that is designed for the treatment of nanoparticles, nanodispersions, nanoemulsions, and cell disruption.

PolyScience’s recirculating chillers and refrigerated circulators control temperature with stabilities as precise as ±0.005 °C for analytical instrumentation.

Ross’ SysCon PLC-based Control Systems are designed for sequential control of automated processes including mixing, pumping, chemical dosing, water treatment, fractionation, and heat exchange.

Richard Grant, Global VP of Cell Therapy at Invetech discusses the advantages and challenges of developing and manufacturing personalized immunotherapies.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses good engineering practices.

The fast growth of the global biopharmaceutical market has prompted global pharmaceutical and biotechnology companies to increase their R&D investment in biologics.
While the optimization of a lyophilization cycle for a biologic relies on a well-characterized formulation, viscosity and aggregation after product reconstitution must also be carefully managed.

Protecting workers, patients, and the environment requires advanced technologies.

A new technical report guides bio/pharma companies in establishing a risk-based approach for prevention and management of drug shortages.

Sessions address cell therapies, tableting, continuous processes, serialization, and more.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

While the skin offers an alternative route of administration for local and systemic drug delivery, developing semi-solid dosage forms can be a challenge.

Cleanability is crucial when choosing components for GMP manufacturing areas.

The pharmaceutical manufacturing platforms of fluid-bed granulation and milling are widely used to modify particle size. However, the adoption of process analytical technology to monitor and control these processes is difficult because of their dynamic nature. This study examines the efficacy of a particle characterizing technology to capture particle images under dynamic conditions and to calculate particle size distribution data both in-line and at-line during fluid-bed granulation and milling.
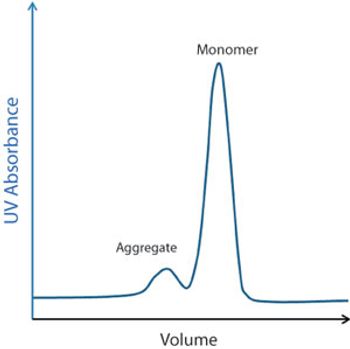
Several chromatographic resins are available for downstream purification.
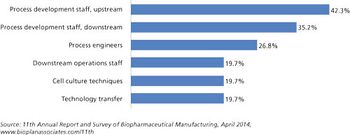
Is there enough talent to go around?

A General Chapter on mAbs will be published in USP-NF as biologics increase their role in healthcare.

Click the title above to open the Pharmaceutical Technology March 2015 issue in an interactive PDF format.