
A reference book omits important information and ignores advanced testing procedures.

A reference book omits important information and ignores advanced testing procedures.

The economic case for personalized medicine offers a solution to the industry's recent woes.

Essential components of a good inspection: good ingredients, proper inserts, and ... deer?

A changing regulatory environment is on the horizon for excipient suppliers and users.
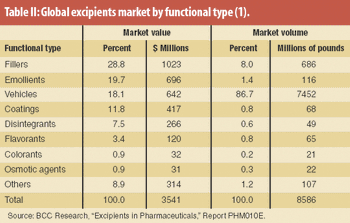
Moderate growth is projected for the global excipients market. Excipient producers target blends and new grades for improving functionality and performance.

Although there have been recent setbacks for China with regard to exported products, the country has made substantial strides in its pharmaceutical industry over the past few years. Intense competition and a series of new regulations seem to be moving the country into a period of recovery.

The recent heparin contamination event calls for a new approach to manage a lengthening pharmaceutical supply chain.

The authors developed a reliable spectrophotometric method for determining and measuring trace amounts of lead in various samples.

The FDA itself issues a cry for help. Is anybody listening?

Excipient producers and industry observers share their perspectives on innovation for excipients.

Antibodies are highly specific molecules that can be tailored to recognize almost any stretch of peptide that nature can conjure: a feature that has been exploited for years now to produce therapeutic antibodies.

Sophisticated replication techniques have made counterfeiting and fraud a serious threat to the pharmaceutical industry.

Regulatory agencies expect companies to establish and monitor clean equipment- and dirty equipment-hold times for manufacturing equipment as part of their cleaning validation program.

Was FDA's decision to issue a draft guidance on of-label information, amidst Congressional scrutiny, the right thing to do?

The annual show provided one-stop shopping for packaging equipment and materials.

Companies continue to develop inhaled insulin and other drugs, despite the problems that Pfizer's "Exubera" experienced.
Manufacturers expect to see the latest developments in process equipment at INTERPHEX, and this year's show was no disappointment. Exhibitors regularly display additions to their lines of encapsulators, tablet presses, material-handling machines, and other automated manufacturing equipment. But the products on view in Philadelphia Mar. 26–28 were not limited to manufacturing applications.

Undertaking process validation involves a major commitment in terms of personnel, resources, time, and money. Performing prerequisite verifications can reduce the risk of making costly mistakes. This Part 1 article explains the value of performing prerequisite verifications and presents case-study examples and real-world solutions to avoid costly process validation failures.

What makes a drug ripe for respiratory delivery?

Heparin contamination casts a shadow on regulatory oversight of product quality.

Brief pharmaceutical news items for April 2008.