
Eisai and Biogen Idec pursue an innovative approach to capacity management.

Eisai and Biogen Idec pursue an innovative approach to capacity management.
Continuous flow chemistry offers potential for greater control, improved safety and environmental profiles, and efficient chemical transformations.

Latin America's diverse growing market seeks regulatory harmonization.

Contract API manufacturers proceed with select investment in capacity and service additions.
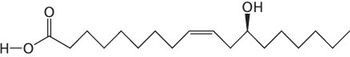
This article summarizes the development and modernization of the United States Pharmacopeia-National Formulary (USP-NF) fixed-oil excipient monographs. This article contains bonus online-exclusive material.

A Pharmaceutical Technology survey shows satisfaction with utility and innovation in most solid dosage and parenteral drug-manufacturing equipment.

The annual INTERPHEX show presents end-to-end packaging solutions.

Industry is moving toward closed-loop control of continuous processing.
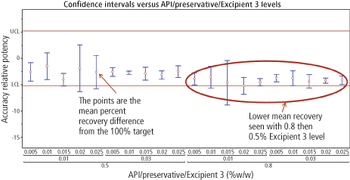
The authors describe a method-validation-by-design (MVbD) approach to validate a method over a range of formulations using both design-of-experiment and quality-by-design principles to define a design space that allows for formulation changes without revalidation.

A Q&A with Michael Lacey of the National Institute for Bioprocessing Research and Training

The miniaturization of preclinical safety assessment studies using a microfluidic chip system and optical microscopy can help reduce compound requirements, time, and costs in formulation development.
Experts describe best practices for sterility assurance in parenteral drug manufacturing. This article contains bonus online-exclusive material.
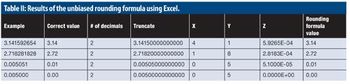
The mysteries of rounding are exposed; strict, unbiased rounding can be applied.

The Thai government is ramping up efforts to promote and develop the biotechnology sector in a bid to enhance its global competitiveness.

Strengthening government control or striving for compliance with international standards?

David Elder, vice-president, technical at PAREXEL, discusses US legislation allows for inspection of generic-drug activities.

USP is proposing a new performance testing standard for moisture permeation of pharmaceutical packaging.

Opioid abuse generates calls for efforts to curb distribution, develop abuse-resistant formulations.

In the context of international trade, the need to develop global quality standards for medicines is increasing.

Sound policies are needed to govern the substitution of interchangeable biologics.

A Q&A with Tony Hitchcock, head of manufacturing at Cobra Biologics.

Conference and exhibits provide a meeting place for professionals to exchange ideas.

FDA's Brian Hasselbalch provides an overview of trends found in FDA quality inspections over the past year.

Latest news about compound pharmacies, biosimilars, prescription drug purchases, and other regulatory topics.

The European Union authorities are stepping up their efforts to incorporate quality-by-design principles into their regulations and guidelines.

Click the title above to open the Pharmaceutical Technology April 2013 issue in an interactive PDF format.