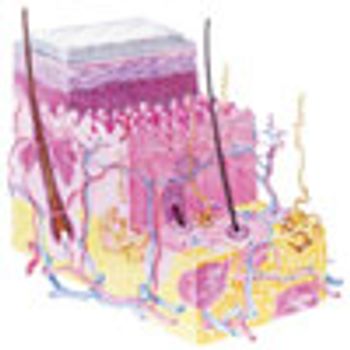
Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

While the United States and Europe still dominate, CMOs and CROs based in emerging markets continue to capture market share.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to keep up with changing regulations.

An integrated pilot plant tests heteronucleation and continuous crystallization.
Adjusting the tablet press and its systems can prevent manufacturing and product quality problems.

The European Pharmacopoeia defines the format and content of monographs for biologicals to keep pace with recent approaches and meet the needs of its users.

Compliance with the new traceability requirements necessitates an understanding of how and when to begin implementing changes in an ever-evolving industry.
The rapid testing of biologic raw materials can lead to greater efficiency.

Matt Hicks, COO and counsel at Federal Equipment Company discusses purchasing used equipment, the potential cost savings, and the strategies companies should consider in redeploying surplus equipment.

Videojet’s 7810 Ultraviolet 2-Watt laser is designed for permanent, high-resolution marking on high-density polyethylene, low-density polyethylene, and synthetic fiber materials.

Ross’ HSM-100LH-3 Vacuum High Speed Disperser is designed for up to five gallons of laboratory and pilot-scale mixing.

Telstar’s compact steam sterilizing autoclave model is designed with hinged front-facing access panels.

Thermo Scientific’s TSX ultra-low temperature freezer is designed to lower its impact on the environment with the use of natural refrigerants, using up to 50% less energy than conventional refrigerant ultra-low freezers.

Transitioning from paper records to electronic batch records decreases costs and increases efficiency.
The author reports results of evaluations and concludes that a disinfectant composed of a low-concentration suspension of silver ions is completely sporicidal with only a one-minute contact time.

Pharma can boast of big-picture successes, but needs to work on operational issues.

The restructuring of the International Conference on Harmonization, which is expected to begin in late 2015, could have a significant impact on the way pharmaceutical regulations are harmonized worldwide.
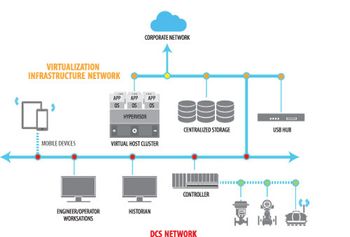
Virtualization has been mainstream in information technology (IT) for decades.


Click the title above to open the Pharmaceutical Technology May 2015 issue in an interactive PDF format.

Drug manufacturers face added pressure and incentives for meeting new FDA compliance policies and priorities.