
Process Analytical Technology

Process Analytical Technology
Researchers have developed injectable, reformable, and spreadable hydrogels capable of delivering sustained release of the proteins they contain for up to six months.

Q&A on GDUFA implementation with Aloka Srinivasan, PhD, a principal consultant with Parexel and former team leader in FDA's Office of Generic Drugs.

A review of taints and odors in the pharmaceutical and consumer healthcare industries.
IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development, with a focus on stability.

PDA's strategic plan calls for maintaining valuable and effective relationships with global regulators.

Advances in targeted drug delivery and customized release profiles are key industry goals.

Packaging and monitoring tools protect temperature-sensitive pharmaceuticals.

Ties between the biotechnology industry and university research are crucial.

The European Commission remains vigilant in monitoring potential pay-to-delay deals.

International trade can be great for business, but breaking border laws can put one in hot water.

Industry experts share perspectives on analytical instrumentation, methods, and data analysis.

Industry and academia advance novel approaches for achieving enanioselectivity.

New product reviews for September 2012, featuring products for manufacturing.

The weak global economy adds to the challenges of bio/pharma companies and their suppliers.

Identifying and classifying rouge can help to determine CAPA.

USP optimizes identification tests and impurities procedures.

Import controls and risk strategies aim to promote quality and spur new drug development.

Brazil takes first steps towards gaining quality requirements for pharmaceutical excipients.

MIT survey results address product and site characteristics that statistically correlate with quality performance.
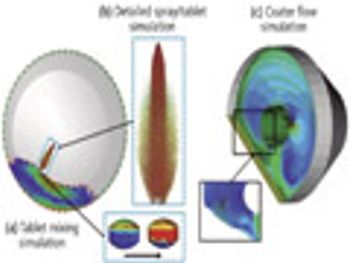
The authors investigate the tablet-coating process using a combination of different simulation techniques.