
There has been a notable shift in interest for life-science companies over recent months from institutional investors investing through the public markets, but any plans to release further capital will rely on first-rate R&D.

There has been a notable shift in interest for life-science companies over recent months from institutional investors investing through the public markets, but any plans to release further capital will rely on first-rate R&D.

New FDA supply chain policies aim to strengthen inspection and oversight processes.

Are strategic partnerships in clinical research a model for CMC services?

I Holland has introduced the next generation to its range of MF polishers, the MF40 automated punch, and die polishing machine.
The adoption of quality by design in small-molecule drug development and manufacturing continues to evolve as the industry seeks ways to augment process understanding for APIs

PDA/FDA regulatory conference promotes a commitment to quality.

The advantages of using an automated powder dispensing system in a ventilated balance enclosure for efficient handling and effective containment of potent compounds are discussed.

The aim of the European Falsified Medicines Directive is to improve the quality of imported APIs, but does the pain now outweigh the gain?

Industry experts discuss the requirements and challenges involved in getting a biosimilar product from bench to launch.

Omega Design Corporation offers the LabelSync 450 Vision Module, designed to capture and sync a bottle?s unique serialized label with its individual line code.

The Bosspak VTC 100 electronic tablet and capsule counter from Romaco?s is designed to fill pharmaceutical solids or food supplements into bottles at high speed.

Restricted access barrier systems (RABS) maximize product control but minimize operator interaction in aseptic manufacturing.
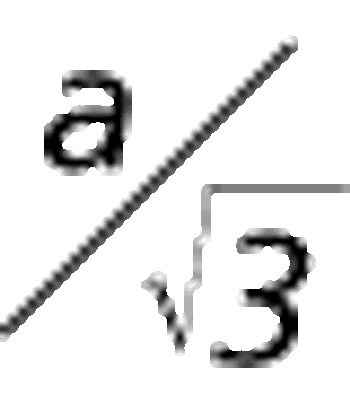
How good is a reportable value?

Pharma eyes biologics production in Brazil as the government begins to recognize the potential of these drugs.

CentuRecon enables dry formulations of therapeutic proteins to quickly be prepared for injection at high concentration.

John Yin, an applications specialist with Freeman Technology, discusses the importance of powder-characterization techniques for optimizing pharmaceutical product development and manufacturing processes.

Establishing a well-defined training program is a crucial activity for any biopharmaceutical organization.
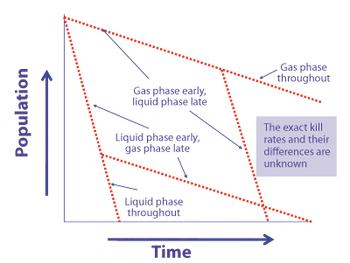
Vaporous hydrogen peroxide, used for sterilization and decontamination, is highly potent but presents implementation challenges.
Developments involve stereoretentive cross-coupling, enantioselective alcohol silylation, strategies for amplifying signals in circular dichroism spectroscopy, and a synthetic route for the natural product ingenol.

Click the title above to open the Pharmaceutical Technology September 2013 issue in an interactive PDF format.