
Transdermal delivery takes up once-forbidden compounds, reviving markets and creating formulation opportunities.

Transdermal delivery takes up once-forbidden compounds, reviving markets and creating formulation opportunities.

Change is inevitable, as is contract manufacturing. But which companies will survive the drifts?

Carl Zeiss MicroImaging (Thornwood, NY) recently introduced its "Colibri" light-emitting diode (LED) light source for fluorescence microscopy. The product is "the only LED light source optimized for white-field microscopy systems," says Becky Homan, product manager for biomedical light microscopy.

Lancaster Laboratories (Lancaster, PA) acquired Microchem Laboratories (Waterford, Ireland). Microchem was established in 1986 and is one of Ireland's testing and research laboratories. Microchem offers microbiological, chemical, and environmental analyses to the pharmaceutical, medical-device, and chemical industries in Europe and Asia.

Amidst debate, the European Pharmacopoeia Commission is working to include physical or "functionality-related characteristics" of exipient materials in its monographs.

When it comes to designing and sourcing sustainable packaging, there are no simple answers. Sustainable packaging depends on a complex interaction of environmental, social, and economic considerations, which are influenced by geography and other factors such as renewability, compostability, biodegradability, weight, and performance.

The United States House of Representatives passed its version of the Patent Reform Act of 2007 (H.R. 1908) in September in a 220-175 vote. Although the bill aims to fix current flaws in the US patent system and to bring it in line with those of other countries' systems, the biopharmaceutical industry is largely unhappy with the news, arguing that it will reduce patent protection.

To monitor and control processes or products, analytical methodology must be fit for purpose. An approach to apply quality by design principles to the design and evaluation of analytical methods has therefore been developed to meet these needs. This article features a downloadable template on which to conduct a failure mode effect analysis (FMEA).

Could sloppy due diligence practices hurt CMOs and their clients?

Our files pull up lost contracts, poor competitive tactics, and an over-ambitious employee.
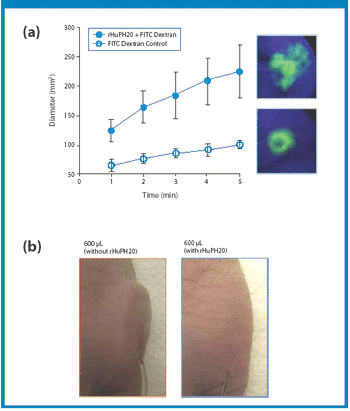
The preferred route of administration for an injected therapeutic agent is subcutaneous (SC), but SC injections are generally limited to no more than 1-2 mL in volume, representing a major challenge, especially for large protein biologics.

Nonpharmaceutical manufacturers find ways to make environmentally sound packaging.

Regulators and industry professionals provide a guide for identifying and measuring impurities.

Courts are denying the terminally ill access to experimental drugs, leaving many with no options.

Offshore contracting is nothing new for the pharmaceutical industry. Neither is importing active ingredients, or prescription drugs, for that matter. But the authorities are having trouble keeping up with these growing international trends.
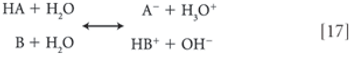
Through consideration of the ionic equilibria of acids and bases, one may readily calculate the formation constant of a salt species solely on the basis of knowledge of the pKA value of the acid and the pKB value of the base.

Orally disintegrating tablets (ODTs) continue to attract attention as an alternative to conventional oral dosage forms.

Expanded access to drugs for seniors has increased demand and focused attention on costs.
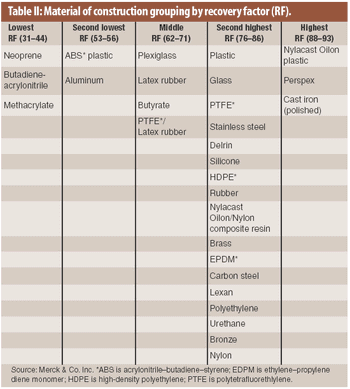
The material of construction is a factor in the recovery of residue in cleaning validation. An analysis of existing recovery data showed that recovery factors for drug products on various materials of construction may be categorized into several groupings.
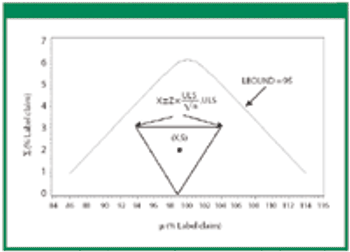
Revisions to the United States Pharmacopeia's (USP) uniformity test require manufacturers to establish new acceptance limits. The authors present their method for calculating acceptance limits consistent with USP's revised content-uniformity test requirements.