
Amie Gehris of Dow Water and Process Solutions discusses formulating abuse-deterrent drugs.

Amie Gehris of Dow Water and Process Solutions discusses formulating abuse-deterrent drugs.

Ireland?s life-sciences sector has grown significantly since the 1960s. To gain insights into the competitive edge that Ireland offers to the pharmaceutical industry, Pharmaceutical Technology Europe spoke with Barry Heavey from IDA Ireland, which is a government agency responsible for attracting pharmaceutical and biotech companies to Ireland.

The pharmaceutical industry grows despite conflict in the Middle East.

Temperature-sensitive pharmaceuticals and biologics depend on a variety of services and technologies to establish, maintain, and verify proper storage and transport conditions.

Kurt Lumsden, Director Client Services at Perceptive Informatics, a subsidiary of PAREXEL, discusses regulatory requirements for the drug accountability process.

Practicality of implementation should be a part of vision in the bio/pharmaceutical industry.

LCMS-8050 Mass Spectrometer Increases Quality Analysis

Recent FDA enforcement activity reveals issues with vial-filling, adequacy of QA/QC procedures, particulate matter in inhalation powders and injectables, and drug labeling.

USP seeks input from stakeholders on new and revised standards to mitigate extractables and leachables in plastic packaging systems.
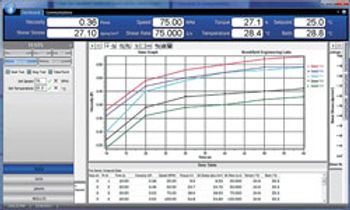
RheocalcT Software Tests Parameters and Data Collection
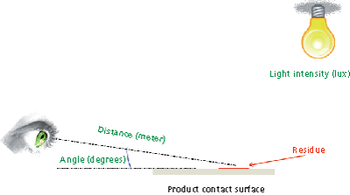
The authors evaluated a variety of materials of construction (MOCs) and found that visible residue limits (VRLs) were higher on some MOCs than on stainless steel. The optimal viewing conditions were dependent on the MOC and the viewing background. The risk of a cleaning failure due to visual failure for different MOCs can be mitigated or eliminated using complementary cleaning validation studies.

Foreign companies zero in on Myanmar with the hope of securing a foothold in its pharmaceutical market.

Video Capture and Synchronisation System
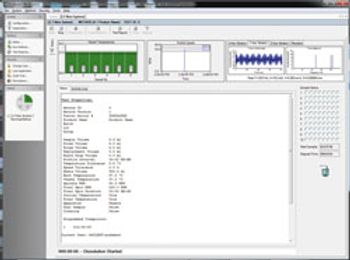
Software Improves Monitoring of Pharmaceutical Ingredients

The authors discuss a strategy for developing a risk-based approach for testing of elemental impurities in leachable studies.

Manufacturing standards are considered key to preventing drug recalls and shortages.

The rapid uptake of single-use vessels for use in bioprocessing applications has made assuring integrity that much more crucial; helium integrity testing can be used to test integrity and prevent failures.

Through its educational and networking opportunities, the American Association of Pharmaceutical Scientists plays an important role in partnering throughout the drug- development and commercialization process.

Industry experts discuss the importance of characterization studies during biosimilars development and related analytical methods.
Scientists from the CDMO Metrics talk about the challenges in developing oral formulations for poorly permeable drugs and the strategies used to enhance oral absorption in the gastrointestinal tract.

Analytical methods are being used to troubleshoot tablet-sticking problems and to develop screening methods and predictive models to more quickly find solutions.
Fluorinated molecules play an important role as pharmaceutical compounds. Recent advances seek to overcome the challenges of selective and late-stage insertion of fluorine into small molecules.

Click the title above to open the Pharmaceutical Technology October 2013 issue in an interactive PDF format.