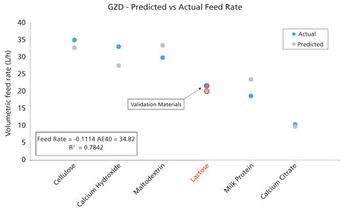
Multi-faceted powder characterization, including measurement of dynamic properties, can be used to correlate powder properties and process performance to support equipment selection, optimization, and troubleshooting.

Multi-faceted powder characterization, including measurement of dynamic properties, can be used to correlate powder properties and process performance to support equipment selection, optimization, and troubleshooting.


Manufacturers of parenteral drugs face challenges to increase efficiency, control particulates, control extractables and leachables, and eliminate product/package interactions; new containers and packaging equipment offer increased options.

The complexity of new packaging regulations laid out in the Falsified Medicines Directive could threaten the existence of smaller pharma and packaging companies.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss performing investigations of biological products.
Safer solid reagents and new coupling chemistry are important developments.

This article looks at the current status of alcohol-induced dose dumping of modified-release formulations and the need for regulatory harmonization in handling this challenge.
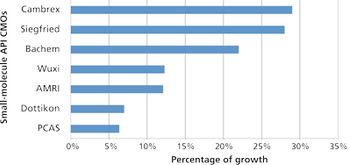
Despite emergence of biologics, small-molecule APIs benefit from industry growth.

The Alpha modular machine platform from Netzsch is designed to mount different grinding systems on the same base platform as appropriate for a defined drive capacity.

GEA’s Pony NS2006L is a self-contained, high-pressure laboratory and pilot plant homogenizer for product development of advanced fluid applications.

The flexible Dolomite Flow Chemistry Systems from Dolomite Microfluidics can be configured to suit different applications.

Ross SysCon’s UL-rated and CE-marked control systems are custom-designed for industrial processes such as mixing, pumping, chemical dosing, dilution, heat exchange, separation, drying, and waste treatment.

In the development of biopharmaceuticals and pharmaceuticals, the line is blurring.

New formulations that enhance bioavailability, optimize drug-delivery profiles, reduce dosing frequency, or improve patient experience have the potential to deliver quicker returns on investments than developing a completely new drug.

Managing change and overcoming employee resistance and fear requires a proactive approach.
An integrated approach can improve the efficiency of cleaning validation studies.

Click the title above to open the Pharmaceutical Technology October 2015 issue in an interactive PDF format.

Manufacturers challenge details in new policies designed to promote access to important therapies.