
The authors describe some cases of container closure design flaws and actions taken by FDA to mitigate safety risks and increase patient acceptability.

The authors describe some cases of container closure design flaws and actions taken by FDA to mitigate safety risks and increase patient acceptability.

This article will equip the excipient vendor with an understanding of QbD from the perspective of the topical pharmaceutical product manufacturer.
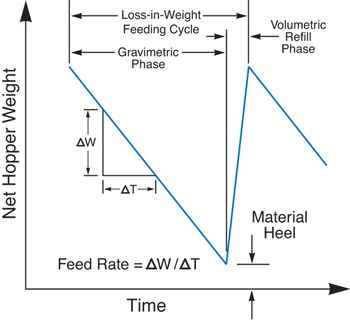
Designing loss-in-weight feeders for accurate and consistent refill is crucial to a continuous solid-dosage process.

Primary packaging and manufacturing technologies minimize product/package interaction, protect quality, support safe travel through the supply chain, and enhance performance at point of use.

Efforts to accelerate drug development will alter fee structure and require ready production sites.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses the assessment of risk in the processing of intravenous injectable drugs.
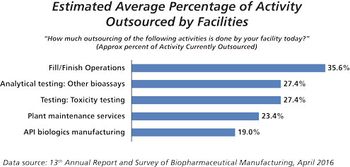
This key bioprocessing segment is expecting continued growth

The editors seek drug development experts to contribute technical articles for 2017.

Excipients play a crucial role in the manufacturing of solid-dosage forms and the performance of the finished drug product.

A number of organizations have analyzed and estimated the size of pharma’s counterfeit and diversion problem.

As criminals duplicate the latest overt security technologies, pharmaceutical manufacturers are evaluating covert and layered approaches to fight counterfeiting, theft, and illegal product diversion.

Researchers develop catalysts that mediate complex transformations under conditions appropriate for commercial manufacture.

Regulators are tightening up on post-marketing monitoring of biological medicines to detect deficiencies caused by manufacturing problems, particularly those stemming from post-authorization changes in the manufacturing process.

The CELLdisc from Greiner Bio-One is a multilayer cell culture vessel that offers a growth area of up to one square meter for adherent mammalian cells.

The Double Planetary Mixers from Ross, Charles & Son are offered in 4-, 5-, 10-, and 25-gallon models for pilot-scale production of highly-filled pastes, dough-like materials, shear-sensitive gels, granulations, and powder blends.

The Print & Check Flex Machine from Antares Vision is a flexible high-capacity serialization unit created for track and trace.

Understanding the components of a reference marketed pMDI is needed to develop a generic pMDI.

The aCOLade 2 Manual Colony Counter from Synbiosis includes automatic count recording.

Click the title above to open the Pharmaceutical Technology October 2016 issue in an interactive PDF format.

Multiple methods are required for detecting and removing protein impurities.