
This position paper describes a model for the future that would provide appropriate standardization, facilitate drug registration and support regulatory agencies.

This position paper describes a model for the future that would provide appropriate standardization, facilitate drug registration and support regulatory agencies.
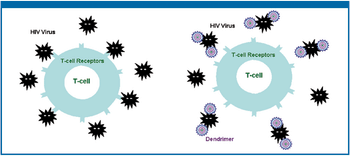
This review provides an update of how dendrimer technology is being applied to the development of novel systems for various topical delivery applications.

Policymakers weigh new rules to ensure the safety and quality of drugs made with tiny particles.

Optimizing the solid form of a drug reaps scientific and technical awards.

A well-balanced guide to industrial bioseparations provides valuable information.

Brief pharmaceutical news items for November 2008.

Data capture needs to be fast and reliable...so which automatic identification technology is best?

Attendees at a recent workshop endorsed new methods to detect metals in drugs, dietary supplements, and food ingredients.
With government support, China's pharmaceutical equipment sector is trekking ahead despite challenges regarding the country's overall perceived product quality.

The Indian government may soon monopolize its pharmaceutical industry to cut costs and improve healthcare, but the move is sounding off alarm bells with the companies whose drug products are under review.

The US Food and Drug Administration seeks to understand nanotechnology better and exercise appropriate oversight over products that incorporate it.
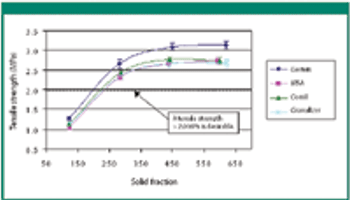
The authors evaluate the effect of various mill types on particle-size distribution, flowability, tabletability, and compactibility.

Despite challenges, contract manufacturers in Europe are enjoying considerable success.
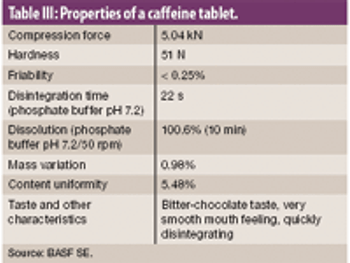
The authors examine the effectiveness of an excipient comprised of mannitol, polyvinyl acetate, and crospovidone using model actives loperamide hydrogen chloride and caffeine.
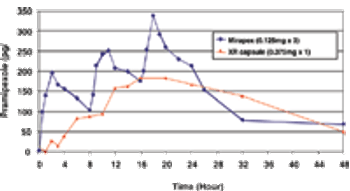
Novel hydrophobic bioadhesive polymers and dosage designs are now available to effectively achieve tailored release kinetics of a broad range of drugs to meet the clinical needs.

Pharma companies could benefit from the lessons learned in this fall's financial crisis.
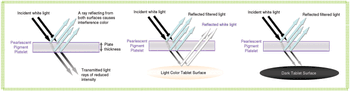
Sophisticated excipient development, especially for coatings, is staying on top of new challenges and meeting expanding industry needs.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Production problems come in all shapes, sizes, and ... species.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.

Pharmaceutical Technology will feature video coverage of AAPS this month.



Molecules called "chaperones" facilitate correct protein folding.