
A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

Contract organizations waiting for the pipeline to come roaring back are kidding themselves.

New tests solve one issue, but cheaper plastic and new stoppers cause problems.
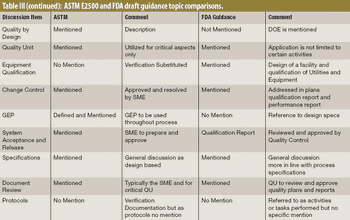
The author explores differences between two qualification documents, the draft guidance from FDA "Process Validation: General Principles and Practice" and the ASTM E2500-7 standard "Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment."

The heightened focus on risk raises concerns about delays in approving new drugs.

With a five-yar revision cycle around the corner, USP will hit or miss the collaboration mark.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

New standards may overlook critical qualification needs.

Functionalized supramolecular catalysts and an enantioselective route to unnatural amino acids are some recent developments.

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.

The authors developed a robust, automated system to conduct Karl Fischer moisture assays for lyophilized products.

Engels discusses the latest industry developments and trends.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.
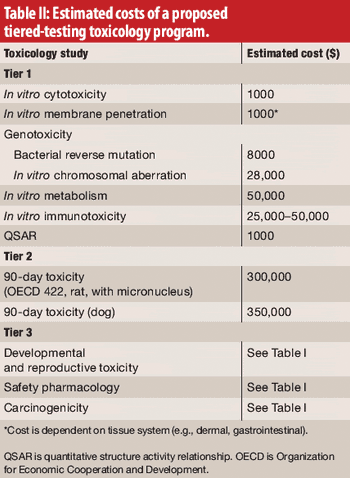
The authors, representing the International Pharmaceutical Excipients Council, propose a new evaluation procedure, including tiered toxicology testing for excipients.

Editors' Picks of Pharmaceutical Science & Technology Innovations

New pricing controls and healthcare reforms may be pushing the pharmaceutical market out of this southeast Asian country. This article contains bonus online-exclusive material.

Sharing Supply-Chain Security