
VIDEOJET’s thermal ink jet printer, the Videojet 8610, is designed for high-resolution coding with MEK-based fluids on non-porous packaging, including films, foils, plastics, and coated stocks.

VIDEOJET’s thermal ink jet printer, the Videojet 8610, is designed for high-resolution coding with MEK-based fluids on non-porous packaging, including films, foils, plastics, and coated stocks.

Sartorius Stedim Biotech’s BIOSTAT A is a compact bioreactor and fermenter with accessible peristaltic pumps, probe ports, and supply connections.


Professionals share the ups and downs of working in the bio/pharma industry.

Operational changes at FDA and CDER aim to improve global market monitoring.

Fitzpatrick’s high-containment roll compactor, the Contained Chilsonator System, offers accurate force measurement, optional vacuum deaeration system, increased downstream powder flow characteristics, and a sealed design to decrease air-borne dust.

The spate of drug scandals may alter the relationship between manufacturers and research institutions, and reshape Japan’s clinical research industry.

Dual sourcing is one of many possible solutions to securing the supply chain.

Bio/pharma employees say they are more secure in their jobs, but may seek better opportunities with other employers.

FDA approves treatments for new diseases and drugs that operate by new mechanisms.

On Oct. 7, 2014 at CPhI Worldwide in Paris, France, UBM Live announced the winners of the 11th annual CPhI Pharma Awards.
In anticipation of this mandatory switch from Oxyfume 2000 to 100% EtO for BI testing, comparison studies were performed to determine if the switch from Oxyfume 2000 to 100% EtO would have any impact on BI resistance label claims.

The authors explore and define common industry approaches and practices when applying GMPs in early development.

The author proposes a quality management system that uses the power of executive management to promote a positive quality culture.

Solving the problem of tablet spots or specks involves prevention and thorough investigation.
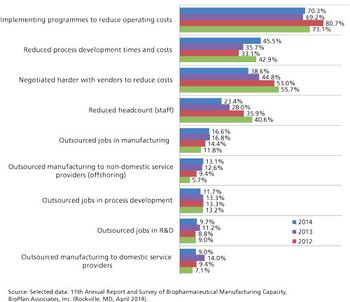
Outsourcing is taking on a greater role in the biopharmaceutical manufacturing industry.

Dr. Thomas Hein, Director Business Development and Regulatory Affairs at Hermes Pharma, discusses the difference between user-friendly dosage forms and conventional tablets and capsules.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the requirements for good distribution practices.

Click the title above to open the Pharmaceutical Technology December 2014 issue in an interactive PDF format.