
Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.
Biosimilars may be the key to CMO growth.

Airlocks, gowning rooms, and transition spaces have different uses and should be considered separately in cGMP pharmaceutical facility design.
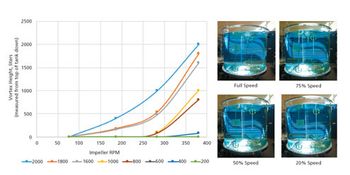
Quantitative and qualitative tools allow better understanding of mixing in a single-use bioprocessing system.

The R Series Shrink banders from PDC International Corporation perform label and neck banding functions at line speeds of 400 to 1000 cpm.

The Prominence Ultra-Fast Preparative and Purification Liquid Chromatograph (UFPLC) from Shimadzu enables fast recovery of highly purified target compounds from complex samples including organic synthesis reaction mixtures and natural products.

Zeiss’s Axio Observer inverted microscope platform includes new automation features, allowing researchers to perform multimodal imaging of living and fixed specimens.
Hot-melt coating was used to develop taste-masked orally disintegrating granules of acetaminophen and caffeine.

Pharmaceutical companies must see regulators as partners in their efforts to provide safe and effective therapies worldwide.

Pharmaceutical Technology reached out to the US Pharmacopeial Convention (USP) to get an understanding about how pharma manufacturers can get involved in developing industry standards.

Susanne Keitel, director of the European Directorate for the Quality of Medicines and Healthcare (EDQM), discusses the role industry plays in the development of pharmaceutical standards.

Better co-ordination within and between regions is needed to improve the global regulation of medicines, according to the European Medicines Agency.

Non-precious-metal catalysts are increasingly employed for commercial API synthesis.

Rookie API developers beat pharma at its own game.

Survey reveals optimism, discontent, and a desire for better career opportunities.
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

The Uniformity of Dosage Units test is used to evaluate content uniformity or weight variation or dosage forms such as tablets and capsules. The author introduces two different acceptance value limits (n = 10 and 30) in this article.

Republican control of Washington promises overhaul of healthcare and medical product regulation.

The Portable High Shear Mixing System from Ross, Charles & Son is designed for powder dispersion into liquid, emulsification, and homogenization in a closed, temperature controlled vessel.

Europe-based bio/pharma employees are unified on satisfaction with employment conditions-and dissatisfaction with salaries.

Click the title above to open the Pharmaceutical Technology December 2016 issue in an interactive PDF format.
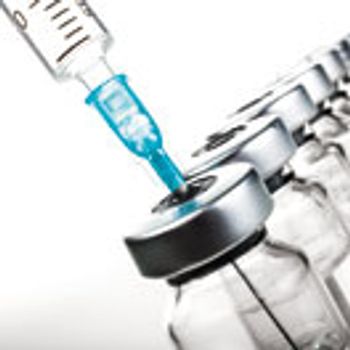
Improvements to aseptic manufacturing procedures are long overdue. But how feasible is it for manufacturers to modernize fill lines of legacy products?