
The biologic is a novel bone builder that has a dual effect of increasing bone formation and reducing bone loss.

The biologic is a novel bone builder that has a dual effect of increasing bone formation and reducing bone loss.

GE Healthcare Life Sciences and Advanced Solutions Life Sciences (ASLS) are partnering on 3D-printing of tissues that can be used for discovery and cytotoxicity testing.

The new Technology Excellence Center, located in Boston, MA, will further the company’s presence in the United States biopharma drug development landscape.

The new technical service center at the Memphis International Airport will service inbound and outbound traffic for the company’s CSafe RKN and CSafe RAP active container systems.

The expansion will enhance the company’s specialty drug product capabilities.

The new software lets users choose the volume and cell concentration from initial cell preparation through transduction to final resuspension, without compromising cell viability and virus stability.

The app, CURE ID, is designed to allow the clinical community to report their experiences treating infectious diseases with novel uses of existing FDA-approved drugs.

The agency has approved three applications for generic versions of Gilenya (fingolimod), Novartis’ blockbuster multiple sclerosis drug.

Cambrex reports that the acquisition by the investment group will facilitate ongoing growth.

Expert poll highlights strength of US pharma sector and advances by emerging markets.

A drug discovery company based in the United Kingdom, Lunac Therapeutics, has been awarded funding worth £3.14 million (US$4.11 million) under Innovate UK’s Biomedical Catalyst program.

Catalent and Ethicann Pharmaceuticals have announced a partnership aimed at developing a new combination pharmaceutical-grade CBD and THC product to treat MS spasticity using Catalent’s orally disintegrating tablet technology.

BioMed X has announced the successful completion of its first joint research project in the fields of COPD and IPF that it had undertaken with Boehringer Ingelheim.

Data released by the Cell and Gene Therapy Catapult (CGT Catapult) have shown that demands for specialist skills and investment in the cell and gene therapy industry in the United Kingdom are set to increase in the near future.

CPI has partnered with ImmunoBiology (ImmBio) for the development of a heat-stabilized formulation of a mutli-antigen vaccine candidate against Pneumococcal diseases.

Iontas has entered into a collaboration agreement with Adaptate Biotherapeutics for the generation and optimization of antibodies for novel immune-oncology targets.

GE Healthcare Life Sciences and Guangzhou Development District Investment Promotion Bureau have signed an agreement to jointly establish a training center for biopharmaceutical professionals.

Horizon Discovery has announced its partnership with the Human Protein Atlas, which will see the incorporation of Horizon’s CRISPR-edited knockout cell models into the Cell Atlas program.

The new 50,000-square-foot facility will create 200 new jobs and accelerate commercialization of gene and cell therapies.
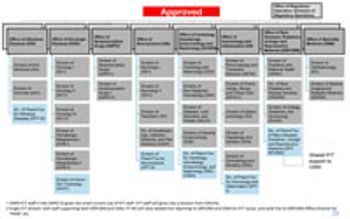
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

The acquisition aids in the expansion of the Astellas Focus Area approach, which involves the creation of medicines for diseases with high unmet medical needs by identifying combinations of biology, therapeutic modality and technology based on emerging science.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

Aiming to break the bottlenecks that are slowing commercialization of innovative therapies, a new $50-million center in Boston will develop both cell and viral vector products within a single facility.

Nearly four decades after the first diagnosis, the fight to treat HIV/AIDs continues.

As the UK heads to the polls for an unusual December general election, industry issues manifestos on medicines while Europe rejoices some movement … finally.

Regulators are facing huge challenges on how to deal with the digitalization transformation occurring in the healthcare and pharmaceutical sectors.

The new treatment is the first cream-based fixed dose combination of calcipotriene and betamethasone dipropionate for topical treatment of plaque psoriasis.

The agency sent warning letters to 15 companies for illegally selling cannabidiol products.

Through the program, the company will introduce seven new manufacturing sites that will be completed and operational by the end of 2020.

Ken Langhorn, who joined the company in February 1998, will assume the position.