
EDCTP has granted EUR10 million to a collaboration of African and European antimalarial drug researchers to support late-stage clinical trials of a novel antimalarial combination.

EDCTP has granted EUR10 million to a collaboration of African and European antimalarial drug researchers to support late-stage clinical trials of a novel antimalarial combination.

Sandoz has signed an agreement with Shionogi for the commercialization rights of Rizmoic (naldemedine) within specific key European markets.

Novartis has announced that FDA has accepted its biologics license application (BLA) for brolucizumab (RTH258) for the treatment of wet age-related macular degeneration (AMD).

EC has granted approval of Pfizer’s VIZIMPRO (dacomitinib) as a monotherapy for the first-line treatment of adult patients with advanced or non-small cell lung cancer (NSCLC).

Former US Senator Jeff Flake and Brandon Allgood, PhD, will be the keynote speakers at the 2019 installment of CPhI North America, in Chicago, IL.

Sharpless will seek further solutions to the opioid crisis and work to reduce cigarette use in adults and kids.

The program and speakers for CPhI North America’s third Women in Leadership Forum have been announced, happening on Thursday, May 2 from 8:00am–11:00am in Chicago, IL.

The companies will merge to offer a broader range of analytical data packages and regulatory support for biopharmaceuticals.

Hovione Technology has acquired global rights to a dry powder inhaler for pulmonary drug delivery, the Papillon DPI invented by Dr. Klaus-Dieter Beller.

FDA sent a warning letter to RIJ Pharmaceutical LLC after an inspection of the company’s Middletown, NY facility found CGMP violations.

The agency sent a warning letter to Luen Fook Medicine Sdn., Bhd. for CGMP violations found at the company’s Malaysia facility.

The partnership includes a $10,000 donation to fund Illinois Biotechnology Innovation Organization (iBIO) STEM programming for girls in grades three through eight.

Catalent’s acquisition of Paragon Bioservices will provide expertise and in expanding gene and cell therapy markets.

EMA is evaluating the safety of Lemtrada (alemtuzumab) after new side effects were reported.

At INTERPHEX 2019, CEO Richard Johnson highlighted the importance of PDA’s new Asian business unit and outlined the organization’s plans. Data integrity guidance for manufacturing and quality systems will be published by the end of the year, as efforts move into big data and artificial intelligence.

Patient-centric drug development is becoming more important in the bio/pharma industry.

Brexit has the potential to rumble on until Oct. 31, 2019 as the UK is granted a further six-month extension by European Union leaders.

Legacy Pharmaceuticals has entered into a collaboration with SCHOTT to solve a technical challenge of leaching that is occurring with an antiviral drug.

NICE has not recommended the use of Novartis’ Kisqali (ribociclib) in combination with fulvestrant for the treatment of hormone receptor-positive, HER2-negative, advanced breast cancer.

Shareholders have approved the issuance of Bristol-Myers Squibb common stock for the pending $74-billion merger with Celgene.

The companies will join forces on a research project to develop end-to-end workflows for the preparation, characterization, and monitoring of biotherapeutics using liquid chromatography-mass spectrometry.

The new service offers a flexible, customizable suite of products and services designed to accelerate the clinical and commercial development of gene and cell therapies.

The acquisition of CiVentiChem’s facility in the United States is expected to enhance UK-based Sterling Pharma Solutions’ chemistry development capabilities.
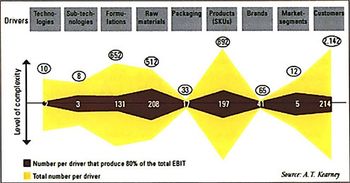
Novartis, BMS, and other companies are reducing complexity by thinning out product portfolios. Reducing complexity can reduce waste and improve responsiveness, but it must be done right.

The European Medicines Agency weighs in on the role of regulators in determining added benefits of novel therapies.

The UK’s Centre for Process Innovation is collaborating with partners GSK and AstraZeneca to establish a continuous wet granulation manufacturing facility for small-scale development of oral solid-dosage pharmaceuticals.

FDA Commissioner Scott Gottlieb and Janet Woodcock, director of FDA’s Center for Drug Evaluation and Research, expect shortages to ease within six months. Although recycled solvents and materials are a prime concern, questions remain about the sources of contamination.

The agency approved Dovato (dolutegravir and lamivudine), as a complete regimen for the treatment of HIV-1 infection in adults with no antiretroviral treatment history and with no known or suspected substitutions associated with resistance to the individual components of Dovato.

Empty pharmaceutical capsules provider ACG aims to merge science and art with its new art competition worth $5000.

Catalent invests $5 million in hot melt extrusion capabilities at Somerset, NJ facility.