
A new report gives an overview of the work of the International API Inspection Program.

A new report gives an overview of the work of the International API Inspection Program.

Kemiex trading network is designed to increase safety in the sourcing of APIs and additives.

This workshop, to be held in Sheffield, United Kingdom, on June 7, 2018, will provide an introduction to the use of glass as primary pharmaceutical packaging.

Innomech has developed an innovative new test station for Fluidic Analytics to streamline R&D programs. The new test station serves as a labor-saving R&D tool to help process multiple test samples and to ensure product quality.

Pharmaceutical Technology will host a panel discussion focused on early drug development on April 24, 2018 at CPhI North America.

A $5.5-million expansion at its Philadelphia, PA clinical supplies facility gives Catalent additional packaging and storage capacity.

The company will build a high-volume manufacturing facility for their glass packaging product in Durham County, NC.

Premier Pharmacy Labs is voluntarily recalling multiple products because of the potential lack of sterility assurance.

Thermo Fisher Scientific and the University of Pittsburgh have established a new pharmacogenomics center of excellence to support translational research that demonstrates the value of PGx in precision medicine.

The contract development and manufacturing organization will discuss its capabilities in topical drug products at CPhI North America, taking place April 24–26, 2018 in Philadelphia, PA.

The new facility, to be built in Toronto, Canada, will significantly increase capacity for pediatric and booster vaccines.

ReForm Biologics and KBI Biopharma will collaborate to improve biopharmaceutical formulations and development.

FDA sent a warning letter to Tris Pharma Inc. after investigators found the company had failed to properly investigate batch failures and establish quality control procedures.

The agency has assigned new EU member state rapporteurs and co-rapporteurs to medicines previously assigned to the UK’s Medicines and Healthcare products Regulatory Agency.
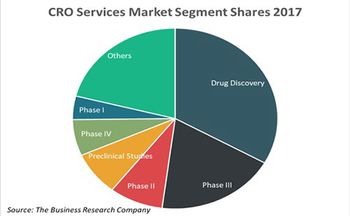
A new study by the Business Research Company reveals prominent contract research organization outsourcing trends.

The biopharmaceutical firm has chosen Rhode Island as the site of its next-generation biomanufacturing plant, which will offer flexibility, speed, and efficiency.

David Weingarten, PhD, and Mark Feldstein, PhD, will present ways to optimize pharma patent protection to avoid generic competition and increase return on investment at CPhI North America 2018.

Serialization technology and implementation expert Rick Seibert will present an insight briefing on recent serialization challenges at CPhI North America in Philadelphia, PA.

The new 300,000-square-foot facility is considered the largest dedicated cell and gene therapy manufacturing facility with fully integrated services.

Pharma event announces plans to alternate locations between Indonesia and Thailand to open new market opportunities.

The manufacturer of glass, electronic displays, and chemical products will showcase fluorinated molecules for pharmaceutical products at the CPhI North America 2018 Exhibition.

The acquisition is expected to accelerate progress towards personalized cancer healthcare.

Roche has acquired a program to develop regenerative therapies for multiple sclerosis.

Under this global collaboration, the companies will develop encapsulated cell therapies for treating Type 1 diabetes.

Celltrion received complete response letters from FDA for its rituximab and trastuzumab biosimilars.

National Institutes of Health researchers use genomics to show that squamous cell carcinomas differ from other cancers, which could advance treatments for head and neck and other cancers.

Anthony Qu, PhD, vice president of Scientific Affairs at Halo Pharma, will give a presentation on fixed-dose combination products, drug products containing multiple active ingredients, as an effective approach for simplified dosing at CPhI North America on Wednesday, April 25, 2018 in Philadelphia, PA.

Boehringer Ingelheim and OSE Immunotherapeutics have entered a global immuno-oncology partnership to develop a checkpoint inhibitor for treating advanced solid tumors.

CPhI North America 2018 is hosting a forum for female pharmaceutical professionals to network, collaborate, and share their perspectives at this year’s conference.

The contract manufacturing organization’s facility in Boulder, CO, has passed general inspection from FDA.