
FDA emphasizes the surveillance aspects of quality metrics to concerned drug manufacturers.

FDA emphasizes the surveillance aspects of quality metrics to concerned drug manufacturers.


FDA has approved ixazomib, the first approved oral proteasome inhibitor.

The University of Sheffield has appointed Cobra Biologics to advance novel fusion protein technology into Phase 1 clinical trials.
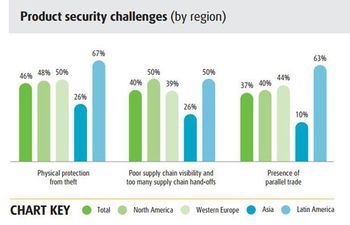
UPS’ 2015 “Pain in the Chain” survey suggests that pharma companies are getting better at product protection, cold chain and regulatory compliance, but need to improve cost control and planning for unexpected events. Lack of transparency and “too many handoffs” remain major challenges.

Expansions at Catalent’s Kansas City, MO, and Madison, WI facilities made in response to industry demand.


Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

Charles River Laboratories announces the acquisition of Oncotest GmbH.
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Experts at the ISPE annual meeting describe best practices, including containment and production in classified spaces.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

Merck KGaA announces the completion of the acquisition of Sigma-Aldrich, a St. Louis-based life-sciences and technology company.

The increase in spending is said to be due to increased access to and use of medicines in emerging markets and higher prices for branded, specialty medications in developed markets.

Operations at Catalent’s Beinheim, France, softgel facility were suspended following suspected deliberate action to misplace capsules.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

The agency issues guidance on the labeling of over-the-counter products that contain acetaminophen.

The new executive director of the European Medicines Agency begins appointment.

GE Healthcare Life Sciences and Emerson Process Management collaborate in biopharmaceutical manufacturing processes.


A landmark study by the National Institutes of Health determines that Lucentis is highly effective as a treatment for diabetic retinopathy.

High-dose axalimogene filolisbac immunotherapy will advance to expansion phase.


An ABPI report found a lack of quality candidates for high-skilled roles in areas such as bioinformatics, translational medicine, clinical pharmacology, and pathology.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.

Roche will invest in Switzerland but leave sites in Ireland, Spain, Italy, and the US as it focuses on lower-volume, specialized medicines.

Consort Medical has combined and integrated Aesica’s drug formulation, manufacturing, and packaging capabilities with Bespak’s drug-delivery device design, development, and manufacturing services to create a global single source drug and device contract manufacturing partner for the pharmaceutical industry.

Lawsuit alleges birth control packaging error led to 113 unwanted pregnancies.


Under the terms of the agreement, Daewoong will annually purchase minimum API quantities of erdosteine of approximately EUR 25 million from Edmond Pharma, a Recipharm Group company.