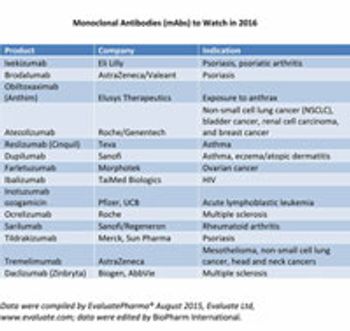
BioPharm highlights the monoclonal antibodies that may gain United States regulatory approval in 2016.

BioPharm highlights the monoclonal antibodies that may gain United States regulatory approval in 2016.

More “me-betters” and more focused breakthroughs could enhance new drug development.

Merck KGaA, Pfizer, and Syndax enter into exclusive agreement to evaluate the use of avelumab and entinostat for ovarian cancer patients.

The revised USP Chapter 1207 gives best practices for obtaining reliable data in container closure integrity testing.

Infrastructure and payer decisions will determine drug choices in emerging and developed regions.

Regulatory, corporate restructuring, and manufacturing issues will challenge bio/pharma to meet the needs and expectations of patients around the world.

Leading nations are backing moves to strengthen WHO’s central role in international health security following the Ebola crisis, which sparked criticisms on the organization’s ability to address a pandemic outbreak.

BASF and Sumitomo Chemical explore in vitro system for chemical safety evaluation.

Valeant announced its CEO, J. Michael Pearson, will be on medical leave of absence after being hospitalized for pneumonia.

Mass Spec Lab, a privately owned analytical company, announced its official launch on Dec. 28, 2015 in Southern California.

Boehringer Ingelheim announced it will establish a new biopharmaceutical production facility in Vienna.

Samsung BioLogics begins construction on their third facility in Songdo, Korea.

GENEWIZ signs definitive agreement to acquire Beckman Coulter's gene services business.

Recipharm has signed an agreement with Sweddish pharmaceutical company LIDDS for the production scale up and manufacture of LIDDS’ Liproca depot for the treatment of prostate cancer.

The aim of the collaboration is to advance the use of Cellectar’s phospholipid drug conjugate platform for targeted delivery of a selection of Pierre Fabre’s cytotoxics.

The National Institutes of Health released a strategic plan covering the fiscal years 2016-2020.

Synpromics announced collaborations with Avalanche Biotechnologies and Applied Genetic Technologies Corporation to use synthetic promoters to develop gene therapies, including adeno-associated virus technology for treating eye diseases.

The agency has published draft guidance on safety assessment for investigational new drug application safety reporting.

Janet Woodcock, director of the Center for Drug Evaluation, highlights FDA's priority list for 2016.

The agency has launched a new web platform to foster scientific innovation.

Martin Shkreli, former chief executive officer of Turing Pharmaceuticals and KaloBios Pharmaceuticals Inc., was arrested on Dec. 17, 2015 at his apartment in New York City for securities fraud.According to a press release from the Eastern District of New York State Attorney’s Office, a seven-count indictment was unsealed on Dec. 17, 2015 in federal court in Brooklyn charging Shkreli with securities fraud, securities fraud conspiracy, and wire fraud conspiracy.

AstraZeneca announced the completion of a tender offer for all of the outstanding ZS Pharma shares.

The new arrangement draws from a consignment approach, in which Walgreens will sell-but not directly own-Valeant’s products.

Through a strategic alliance with WuXi AppTec and a $50 million facility investment, AstraZeneca plans biologics and small-molecule expansion in China.

AMRI adds analytical capabilities to its outsourcing services offerings with the acquisition of Whitehouse Labs.

Quotient Clinical announced on Dec. 16, 2015 that is has acquired Co-Formulate Limited.

Sanofi enters into exclusive negotiations with Boehringer Ingelheim on a business asset swap.

Capsugel adds clinical trial and commercial manufacturing, as well as particle engineering services with two acquisitions.

Researchers from Oregon State University develop a new three-drug delivery system for cancer treatment.