
Novasep gains approval from FDA to produce new molecular entity for US market.

MedImmune and Advaxis partner on immuno-oncology combination clinical trial.

American Health Packaging announced a voluntary nationwide recall of ibuprofen and oxcarbazepine tablets due to mislabeling on the inner unit dose blister packaging.

Unique Pharmaceuticals has issued a voluntary recall of sterile compounded preparations, but aired concerns about FDA?s recall demand.

AbbVie's relocation to Ireland following Shire's acquisition could lower its annual tax expense by as much as 7% over the next 15 years.

AstraZeneca releases designs for its new Global R&D center and corporate headquarters in Cambridge, UK.

CPhI 2014 panel additions to provide global insights from the contract services sector.

After rejecting several bids, Shire has agreed to a merger with AbbVie valued at almost $55 billion.

Alcon enters agreement with Google to in-license its "smart lens" Ttechnology for all ocular medical uses.

Baxter investigates root cause of cellulosic fibers and/or plastics in four lots of IV solutions.

Hovione will use Merrion's GIPET absorption-enhancing technology for solid-dosage drugs.

West's new plant in India will manufacture primary packaging for injectable drugs.

Trifarma cited for significant deviations in data collection and security, and employee training.

Pfizer will acquire InnoPharma for $225 million.

A potential treatment for sickle cell disease has come through the “valley of death” of early-stage development due to support from a collaborative partnership established by the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH).

GlobalData report reveals the US trails Europe in strengthening its stagnant antibiotics pipeline.

New AbbVie proposal results in discussion with Shire about possible $53 billion deal.

Mylan set to acquire Abbott's non-US developed markets specialty and branded generics business in an all-stock transaction.

Glatt and Innopharma Labs have formed a partnership to provide Glatt's customers access to Innopharma Labs' non-invasive, real-time 3D particle characterizer, Eyecon, which has the ability to generate live particle size and shape information.

Sun Pharma, Forest Pharmaceuticals and West-Ward Pharmaceuticals issue recalls over dissolution issues.

Puncture in container and overwrap cause leak in intravenous solution, leading to recall by Hospira.

Baxter Healthcare has initiated a nationwide recall of more than 20,000 containers of a pre-mixed beta blocker due to the presence of particulate matter.

The European Medicine Agency details the agency?s recommendations for drug-marketing authorizations for the first half of 2014.

FDA clarifies GDUFA requirements in regards to abbreviated new drug applications and prior approval supplements.

New International Council on Biotechnology Associations advocates biotechnology growth.

Catalent appoints a new country leader and expands facilities.

Hospira acquires assets from Orchid Chemicals & Pharmaceuticals for $218 million.

The FDA approved Beleodaq for the treatment of patients with peripheral T-cell lymphoma.

FDA grants breakthrough therapy designation to Novartis chimeric antigen receptor therapy.
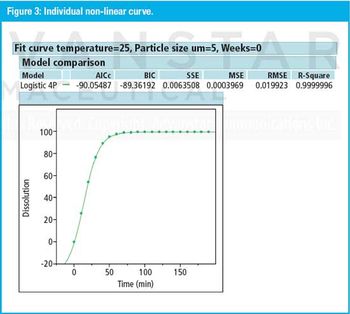
The right approach can provide a clear, statistically defendable method for determining dissolution and accelerated stability.