
Lyophilization Services of New England (LSNE) received a Certificate of GMP Compliance for its New Hampshire facility.

Lyophilization Services of New England (LSNE) received a Certificate of GMP Compliance for its New Hampshire facility.

Adamis Pharmaceuticals agrees to license and potentially acquire 3M's Taper Dry Powder Inhaler technology.

The guidance describes a risk-based approach to monitoring of clinical trials.

A new study reveals how a promising anticancer specifically kills prostate cancer cells by compromising their ability to withstand environmental stress.

Donaldson Company, Inc. announced a $10 million expansion of its testing laboratories at its Bloomington, Minn., corporate headquarters.

Advanced Clinical announced the appointment of Cheryle Evans as vice president, Clinical Operations.

America's biopharmaceutical research companies are developing 444 new medicines to prevent and treat neurological disorders, according to a new report released by PhRMA.

Singapore facility delivers manufacturing redundancy and increases supply assurance for global biologic drug discovery and production.

Weill Cornell scientists reveal how a protein drug eradicated human lymphoma in mice.

Edwards will oversee company's financial, logistical and human-resources operations.

Additional investment from the Biomedical Catalyst will fund multiple programs in the UK.

Selected trendsetters reflect changes in the pharmaceutical industry.

Survey confirms strong link between choice of preferred central laboratory and investigator satisfaction with study sponsors.

A proposed new guideline provides a global policy for limiting metal impurities qualitatively and quantitatively in drug products and ingredients.

Nanomedicines have been authorized by European licensing agencies for more than 30 years but are still posing regulatory difficulties.
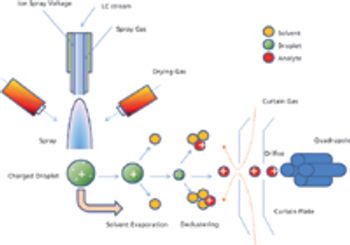
The author describes how liquid chromatography-mass spectrometry works and explains some of its advantages and disadvantages, particularly compared to high-performance liquid chromatography.

The bio-pharmaceutical business outlook in South Korea remains positive.

As the Supreme Court ruled on generic-drug liability, FDA outlined new rules for warning labels.

Orphan drugs for rare diseases are a major area of investment for pharmaceutical companies, but are they becoming too expensive for Europe to afford them?

Bristol-Myers Squibb Company and Samsung BioLogics announced the companies have entered into a 10-year agreement.

A new method of vaccine design, called the Multiple Antigen Presentation System (MAPS), may result in vaccines that bring together the benefits of whole-cell and acellular or defined subunit vaccination.

Wyeth Pharmaceuticals has agreed to pay $490.9 million to resolve its criminal and civil liability arising from the unlawful marketing of the prescription drug Rapamune for uses not approved as safe and effective by FDA.

Perfecseal's high-barrier PVC-free blister-forming web, PerfecForm SkyBlue, is based on polyolefin chemistry, and its new high-barrier flow-wrap specification, PerfecPharm P232, is capable of achieving hermetic seals at the maximum speed capabilities of the top flow-wrap equipment.

Pfizer has announced plans to internally separate its commercial operations into three business segments.

Depomed, Inc. announced that FDA has accepted for filing a new drug application (NDA) from Mallinckrodt for MNK-795.

Aesica has appointed Chris Gowland as Chief Operating Officer, a new position created to further enhance the company's operational performance in APIs and formulated product manufacturing services.

Scientists at A*STAR's Genome Institute of Singapore (GIS) have identified genes that could be potential targets for anticancer agents in the treatment of aggressive breast cancer.

Kemwell Biopharma announced that it has acquired Cirrus Pharmaceuticals Inc., a contract development services company based in Research Triangle Park, N.C.

Australian team develops method for making ultrafine particles for more efficient drug delivery.