
The pharmaceutical majors advance vaccines for the developing world.

Xoma Restructures; AAIPharma Appoints Eric Evans as CFO; and More.

The US Pharmacopeia announced a draft standard containing best practices for ensuring that drugs can be traced to their original manufacturer, are not counterfeited or adulterated, and can be transported to their intended destination without compromising quality.

Novartis Consumer Health has announced a voluntary recall of all lots of select, bottle-packaged configurations of Excedrin, NoDoz, Bufferin and Gas-X Prevention. The recall follows consumer complaints of chipped or broken pills and inconsistent bottle packaging line clearance practices that could lead to stray tablets, capsules or caplets from other Novartis products.

Bristol-Myers Squibb (BMS) has signed an agreement to acquire the clinical-stage biotechnology company Inhibitex in a deal worth approximately $2.5 billion. The transaction will provide BMS with a potential candidate currently in Phase II development for the treatment of hepatitis C.

Ben Venue Laboratories is extending its voluntary suspension of manufacturing at its Bedford, Ohio, facility. The company had originally announced the suspension on Nov. 19, 2011, and announced the extension of the suspension on Dec. 23, 2011.

The National Institutes of Health has established the National Center for Advancing Translational Sciences, a center dedicated to the translation of scientific discoveries into new drugs, diagnostics, and devices.

Alexion Pharmaceuticals has signed an agreement to acquire all the capital stock of the biopharmaceutical company Enobia Pharma in a $610-million all-cash transaction.

USP Releases Guidelines on Ensuring the Integrity of the Pharmaceutical Supply Chain.

AMRI Restructures; ISPE Appoints Nancy S. Berg as CEO; and More.

The FDA has released a new draft guidance that offers recommendations to companies wishing to respond to unsolicited requests for off-label information, including requests made via social media websites.

On Dec. 29, 2011, FDA approved the TIRF REMS Access Program, which is intended to ease the burden on the healthcare system by allowing prescribers and pharmacies to enroll into one new single system instead of several different systems.

The benefits of harmonization may be on industry's wish list, but buying into change is another story.

Recent legal decisions have further divided generic and brand manufacturer cases.

As part of the BRIC bloc with Russia, India, and China, Brazil is one of the world's leading emerging economies and is also considered by IMS Health to be one of seven pharmerging nations, which also include Mexico, Turkey, and South Korea.

Technology may expedite operations, but the absence of the human element could cost dearly.

To keep moving forward, the Pharmacopoeial Discussion Group needs industry participation.

The European Union market takes steps toward continuous processing and modular facilities.

The next two years may see a shake up in the world's current top pharmaceutical companies, with Pfizer likely to be the only US firm to remain in the top five by sales.
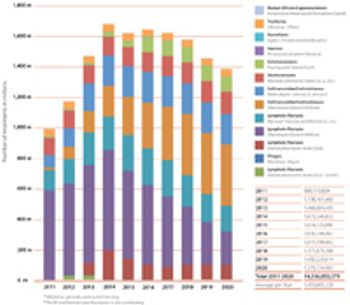
Pharmaceutical companies pledge donations of 14 billion treatments for the next decade to combat nine neglected tropical diseases.

Pfizer and GlaxoSmithKline have announced separate agreements with the GAVI Alliance to supply pneumococcal vaccines to developing countries. Pneumococcal disease can lead to pneumonia, meningitis, and sepsis, and is one of leading causes of death in children under the age of five in developing countries.

AstraZeneca Acquires Chinese Generic-Drug Company; Takeda Makes Management Changes; and More.

On Dec. 21, 2011, Ranbaxy Laboratories signed a consent decree with FDA and pledged that it would strengthen procedures and policies to ensure data integrity and to comply with current good manufacturing practices. The agreement is subject to approval by the US District Court for the District of Maryland.

Last week, Baxter International and Momenta Pharmaceuticals entered into a global collaboration to develop and commercialize follow-on biologic products. The two companies expect to close the transaction during the first quarter of 2012, subject to customary closing conditions.

EMA has unveiled its work program for 2012, which forecasts a slight increase in marketing authorization applications for new medicines compared with 2011. In addition, EMA said that it will, where needed, strengthen the quality, regulatory, and scientific consistency of its assessment process.

The Generic Pharmaceutical Association has proposed a multistakeholder initiative to minimize current and future critical drug shortages. The announcement follows a series of Congressional, executive, and industry response to address the recent problem of drug shortages.

Boehringer Ingelheim Expands Manufacturing Presence in China; Amgen CEO Kevin W. Sharer Announces Retirement Plans; and More.

The Government Accountability Office issued a report recommending that FDA's ability to respond to drug shortages be strengthened. The report was released in conjunction with a Senate hearing before the Committee on Health, Education, Labor, and Pensions on Dec. 15, 2011, on the subject of drug shortages.

Last week, the US Department of Health and Human Services and Novartis Vaccines and Diagnostics dedicated a manufacturing plant that can create influenza vaccine using cultured animal cells instead of the conventional expression system of fertilized eggs.

We have several tablet formulations that are dwell-sensitive-they require more time under compression than other formulations. Given increasing demand, we do not have the luxury of slowing the tablet presses down in an effort to increase that dwell time. How can we maximize dwell time and maintain or increase output in our tablet presses?