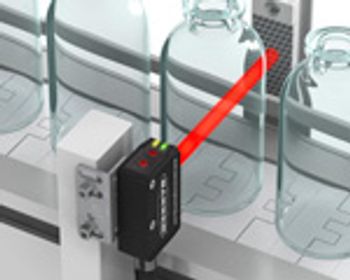
PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the December 2011 edition from Banner Engineering and Eriez.

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the December 2011 edition from Banner Engineering and Eriez.
Sterilization or sanitization is usually applied to kill bacteria in a system. Equipment is cleaned to remove residues from the previous batch of product, and subsequently flushed to remove the cleaning liquids. To ensure that sterilization and cleaning are efficient and safe, it is not enough to develop the appropriate procedures. Selecting the right manufacturing equipment further improves cost efficiency, as well as patient safety.

Risk assessment is not a new concept to the pharmaceutical industry, but lately the phrase has become a mantra. A systemic, science-based way to manage risk is becoming essential to meeting the spirit and letter of FDA requirements.

After reviewing the "shortcomings in quality assurance" that were recently identified at Ben Venue Laboratories's Bedford, Ohio, facility, EMA issued several recommendations, including product recalls for Ecalta and Liminity.

AstraZeneca entered into an agreement to acquire Guangdong BeiKang Pharmaceutical, a privately owned generic-drug manufacturing company based in China, for an undisclosed amount. Upon completion of the acquisition, AstraZeneca will be responsible for the manufacture and commercialization of these medicines.

In a Warning Letter, FDA cited "significant violations" of CGMP regulations, including several repeat observations, at three Novartis facilities located in Colorado, North Carolina, and Canada.

The FDA and EMA are moving from "confidence-building to reliance upon" each other in a step-up in cooperation on GMP inspections; the latest move following successful completion of pilot projects this summer.

Baxter International Agrees to Acquire Synovis Life Technologies; Pfizer Elects President and CEO Ian Read as Chairman of Board of Directors; and More.

Merck Establishes New MSD R&D Asia Headquarters; Astellas Adds Two Senior Executives at Agensys; and More.

Samsung and Biogen Idec agreed to invest $300 million to establish a joint venture to develop, manufacture, and market biosimilars. The deal is Samsung's latest effort to strengthen its position in biosimilars.

On Dec. 6, 2011, FDA announced that a public meeting will be held on Dec. 16, 2011 to discuss recommendations for a user fee program for biosimilar biological products for fiscal years 2013–2017.

Last week, the Pharmaceutical Research and Manufacturers of America Foundation awarded Johns Hopkins University and the University of Washington each a $250,000 grant to establish a three-year graduate certificate program. The program is formally known as the PhRMA Foundation Center of Excellence for a Comparative-Effectiveness Research Educational Program. The funds are the foundation's first grants to educational institutions.

The United Kingdom's life-sciences industry this week welcomed a new, multimillion-pound strategy that aims to both support the industry and encourage innovation in the unsettled economic climate.

In an initiative that could signal a new era in private–public partnerships, AstraZeneca will make 22 compounds available for free to medical researchers next year for projects funded by the United Kingdom's Medical Research Council.

Cultivating a productive investment environment will require partnerships with a range of stakeholders.

Cleanliness is crucial, even if zapping and trapping is necessary to reduce product contamination.

As the excipient supply chain becomes more complex, industry must up the ante to comply with new standards and regulations.

Contract organizations must have highly organized teams and plans to accommodate today's audits.

Political leaders need to consider the impact of the biopharmaceutical industry on the economy.

Packaging is indeed headed to be a lead sector in the Asian pharmaceutical environment, but certain challenges must first be overcome.

Employee training-at all levels-is crucial for moving forward with a successful risk- and quality-based manufacturing strategy.

BioMarin Announces FDA Approval of Expanded Biologics Manufacturing Facility; Par Pharmaceuticals Makes Executive Appointments; and More.

Where does the time go? It is very hard for me to believe that I am already writing for the final issue of the year, looking back over 2011 and planning for 2012.

Elan and the University of Cambridge have launched a center for innovation and drug discovery that will focus on translational research into therapies for Alzheimer's and Parkinson's diseases. The Cambridge–Elan Center will be located at the University of Cambridge, and the agreement between the two will last for 10 years. Its goal is to discover novel compounds that can alter the behavior of proteins associated with neurodegenerative disorders and be developed into new treatments.

EMA Hosts Subgroup Analysis Workshop.

On Nov. 21, 2011, Gilead Sciences agreed to acquire Pharmasset for $137 per share in cash, or a total of approximately $11 billion. Pharmasset's board of directors unanimously approved the transaction, which is expected to close during the first quarter of 2012.

The justice department announced that Merck has agreed to pay $950 million to resolve criminal charges and civil claims related to its promotion and marketing of the painkiller Vioxx.

Despite the fact that Europe's population is ageing rapidly, the region is astonishingly still underprepared for dealing with the health-related effect of this demographic trend.

The breast cancer indication for Avastin has been revoked after FDA Commissioner Margaret A. Hamburg concluded that the drug has not been shown to be safe and effective for this use.

On Nov. 19, 2011, Ben Venue Laboratories voluntarily and temporarily suspended the manufacture and distribution of products made at its Bedford, Ohio, facility. These products include Doxil (doxorubicin HCl liposome injection), which the company produces for Johnson & Johnson. The company's clients include Pfizer, Hospira, and Teva.