
Effective harvesting and purification processes play an essential role in ensuring that biopharmaceutical manufacturing processes provide biologic drug substances with uniform and consistent properties.

Effective harvesting and purification processes play an essential role in ensuring that biopharmaceutical manufacturing processes provide biologic drug substances with uniform and consistent properties.

The collaboration will provide GMP manufacturing ahead of future clinical studies.

A new 20,000-L microbial biologics facility in Ireland will be operational by 2018 for Fujifilm Diosynth's contract development and manufacturing customers.
Process analytical technology paved the way for continuous manufacturing.

Application of flow chemistry for small-molecule API synthesis continues to expand thanks to research efforts.

As the battle for ownership over the use of CRISPR to edit DNA heats up, some of the CRISPR pioneers refocus their efforts to demonstrate the technology’s applications for editing RNA.

Xellia, a generic anti-infective drug manufacturing company, is constructing a laboratory services building at its manufacturing site in Budapest, Hungary.

W.R. Grace & Co. will sell its chromatography product lines, which includes chromatography instruments, columns, and other related products.

A new study in Nature Communications explores how to remove the bulk of the soaps that are added to injectables to make hydrophobic drugs more soluble.

Electrophilic and other reactive compounds, and their precursors, must be measured and removed to ensure patient safety.

AMRI’s acquisition of Euticals expands its API development and manufacturing business.
The production of antibody-drug conjugates requires biopharmaceutical and chemical manufacturing, and conjugation capabilities.
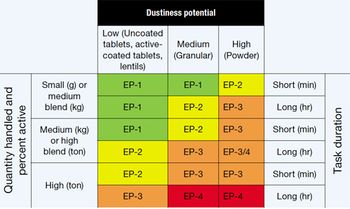
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

Shire announces plans to build a flexible biopharmaceutical manufacturing facility in County Meath, Ireland.

Kollicoat MAE 100-55 from BASF can be used as a direct substitute in commercial enteric pharmaceutical formulations.

Manufacturing highly toxic compounds in a biopharmaceutical environment tests equipment and systems.

A global API marketplace increases the burden of supply chain monitoring for drug companies.

Unsafe material may remain in the US supply chain, according to a March 29th letter to FDA Commissioner Califf

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.

Collaboration will provide for unified development and manufacture of antibody drug conjugates.

Thermo Scientific TruTools chemometrics package extends the capabilities of the portable TruScan RM Raman Analyzer.

The authors present analysis of the state of control of intermediate identity and quality, based on analysis of recently submitted DMFs.

Stephen Hoag, a professor at the University of Maryland (Baltimore), and a member of the National Institute of Pharmaceutical Technology and Education (NIPTE) offers a brief update on issues, and NIPTE’s database project.
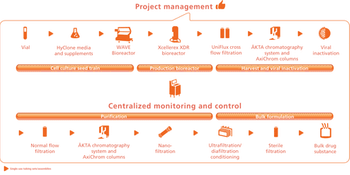
Asking the right questions is crucial to establishing a biopharmaceutical facility design.