
APIs and Excipients
Latest News


Ignoring a contract partner’s ability to handle highly potent APIs (HPAPIs) safely may have serious consequences. Drug owners and contract service providers alike must understand the complexities and liabilities involved in working with HPAPIs.

Competitive pressures are driving more companies to repurpose APIs that had originally been developed for other indications. The industry has only "scratched the surface" of what might be possible, says consultant Hermann Mucke

“Rough notes” documentation and data management failures lead to warning letter for Mahendra Chemicals.

SafeBridge Consultants reports on the status of nine companies in the Potent Compound Safety Certification Program.

Investment group Ardian will acquire the fine chemicals business activities of DPx Holdings B.V.

The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

AMRI expands its API portfolio and European presence through acquisition of Gadea’s Crystal Pharma Group.

Catalent’s licensing of Excelimmune’s antibody combination therapy platform can enable the manufacture of multiple recombinant antibodies in a single batch culture.

Biogen plans to build a biologics manufacturing plant in northwest Switzerland using next-generation technologies to create efficiency and sustainability.
Biologics exhibit greater variability in stability testing than do small-molecule drugs, and maintaining a stable test environment is crucial.
FDA, Congress, and early adopters look to speed up the use of continuous API manufacturing.

GSK will invest in an additional downstream isolation facility for amoxicillin production in Singapore.
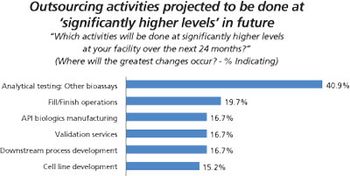
More biopharma companies choose outside service providers for assay testing.

Novasep's new antibody drug conjugate facility at its site in Le Mans, France will be commissioned in 2016.

BioSC Lab is the first in a line of next-generation chromatography equipment for protein purification in batch and continuous modes.

Reach Separations has announced plans to double its laboratory space at its Nottingham facility as a result of increased demand for its specialist purification services.

Monoclonal antibodies can facilitate the entry of radiopharmaceuticals into cells, David Scheinberg said at BIO 2015.

The International Conference on Harmonization finalizes Q&A document on APIs.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.

BPTF seeks changes in performance goals and fee payment schedule in GDUFA renegotiations.

Products on display include the newest addition to the Mobius line of single-use bioreactors; Cellvento CHO cell culture media for optimized production of specific cell lines; and new high-volume AFS water purification systems.

Biopharma companies on both sides of the Atlantic ship more of their assay testing to outside service providers.

Safer reagents and reaction conditions are making many hazardous transformations possible.
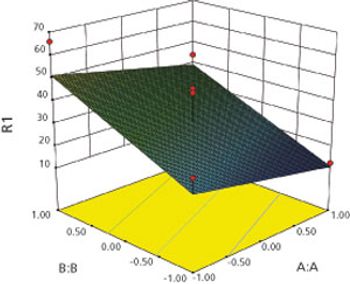
Box-Behnken modeling was used to optimize a resinate complex, to mask the taste of levocetirizine dihydrochloride and montelukast sodium in orally disintegrating tablets.

