
The $10-million investment will include a 500-L single-use perfusion bioreactor and seven patented downstream processing units.

The $10-million investment will include a 500-L single-use perfusion bioreactor and seven patented downstream processing units.

Tergus Pharma announced a partnership with Great Point Partners and construction of a new commercial manufacturing facility in North Carolina.

CDMO iBio introduced cGMP sterile fill/finish services at its facility in Texas.

CDMO, Vibalogics, has revealed that it will be acquired by a private equity firm, Ampersand Capital Partners.

Temporary power, HVAC, and oil-free compressor systems provide flexibility and control for pharmaceutical manufacturing facilities.

Catalent expands gene therapy capabilities with $1.2-billion acquisition of Paragon Bioservices.

The company announced plans to construct a 1.3 million-ft2 integrated manufacturing center in Chengdu, China.

The acquisition of GlaxoSmithKline’s manufacturing site in Cork, Ireland, will expand Thermo Fisher Scientific’s global footprint for complex API manufacturing.
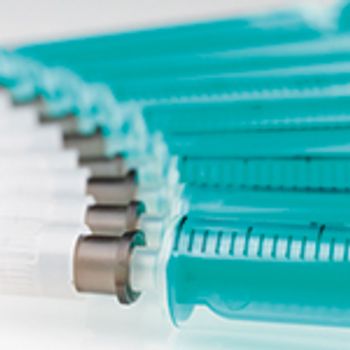
Empty and prefilled syringes must pass a range of quality control tests.

Recipharm Inhalation Solutions is an integrated service for inhalation products.

The investment will increase patient-centric dosage form manufacturing capabilities at Catalent’s Winchester, KY, facility.

Expansion at the Eberbach, Germany facility includes two Vegicap encapsulation lines, printing, inspection, and packaging capabilities.

Cambrex’s expanded quality control laboratory supports generic API development.

The expansion of the CDMO’s Skokie, IL facility includes additional refrigeration and freezer space.

The company passed a seven-day FDA surveillance GMP inspection and announced two upcoming manufacturing partnerships.

The companies form a strategic joint venture for developing and manufacturing live biotherapeutics.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Supplier vetting and monitoring-plus comprehensive testing-ensure quality of raw materials.

Moving from paper-based to digitalized processes is the first step to enabling quality management and manufacturing to work in sync.

Belgium-based CDMO MaSTherCell will expand its European manufacturing capacity for cell and gene therapy products by setting up a new facility in Belgium.

Think ahead to production requirements when planning strategies in early development of gene and cell therapies.

The companies aim to assess automated CAR-T cell therapy manufacturing at the point-of-care and develop technologies to facilitate patient access to immunotherapies.

The Pharma Services business of Thermo Fisher Scientific will invest $150 Million at three facilities.

Alcami Biologics formed to meet market demands for biological drug development services.

Catalent announces investment for its Zydis ODT technology, offering increased drug load and taste-masking capabilities.