
CMOs have been active over the past year in expanding their biologics production and capabilities.

CMOs have been active over the past year in expanding their biologics production and capabilities.

This article provides a sampling of the latest investments, expansions, and acquisitions by small-molecule contract service providers.

Heightened uncertainty means CDMO executives need to play out planning scenarios.

A year’s worth of FDA warning letters suggest that API and finished drug manufacturers should strengthen their approach to continued process verification.
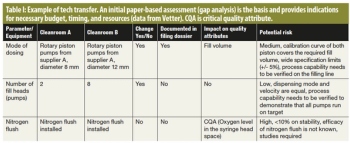
Real-life examples illustrate how to reduce the risks for each transferring partner and ensure that the development process meets regulatory requirements.

The glass and chemical provider will expand its synthetic pharmaceutical intermediate and API production capacity at its plant in Chiba, Japan.

The acquisition will place Cambrex into the finished dosage form CDMO market.

CELLforCURE will produce cancer CAR-T treatments for Novartis at a manufacturing facility in Les Ulis (Essonne), France.
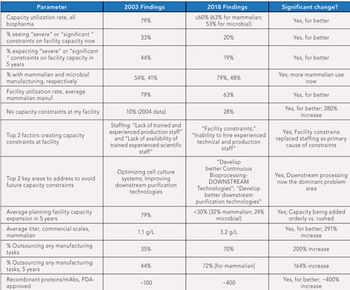
This article highlights 15 years of changes in biopharmaceutical manufacturing.
Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.

Minakem’s facility in Belgium enhances capacity to scale production of highly potent ingredients for small to full GMP batches.

The contract development and manufacturing company has received an additional approval from Health Canada to manufacture monoclonal antibody drug substance at its first plant in Icheon, South Korea.

WuXi STA supported the development of hepatitis drug through marketing authorization holder pilot program.

Recipharm adds inhalation drug manufacturing capacity with the acquisition of Sanofi’s Holmes Chapel, UK site.

Cambrex expands its generic API research and development capabilities at its Milan, Italy site.

Vetter anounced the Open Innovation Challenge to examine the applicability of digital trends to injection systems.

Under the contract, AMRI will focus on the development and manufacture of APIs and drug product for Phase I clinical studies.
From separation systems to reactor technology, new tools are increasing the feasibility of continuous API production.

TxCell announces manufacturing agreement with Lonza for its HLA-A2 CAR-Treg cellular product.

Cambrex invests $5 million in new laboratory expansion at its Karlskoga, Sweden facility.

FUJIFILM Diosynth Biotechnologies opens Cambridge, MA center to promote collaboration with biotech companies.

Rentschler Fill Solutions and Ultragenyx announce fill and finish agreement for the US commercial supply of Mepsevii.

Rommelag will showcase its blow-fill-seal technology that collectively offers container production, aseptic filling, and container closure.

GE Healthcare and the Centre for Commercialization of Regenerative Medicine (CCRM) will support scale-up efforts by DiscGenics for a new cell therapy intended to treat back pain.

This marks the third FDA approval for the company’s second biomanufacturing plant in Incheon, Korea.