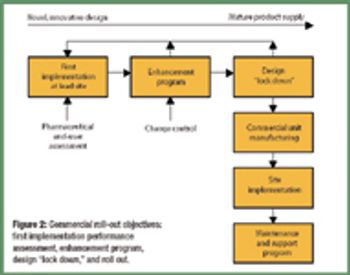
Several problematic issues are associated with the development and installation of automated drug manufacturing and testing processes. The author addresses how vendors can overcome these factors to deliver robust automation across multiple sites.

Several problematic issues are associated with the development and installation of automated drug manufacturing and testing processes. The author addresses how vendors can overcome these factors to deliver robust automation across multiple sites.
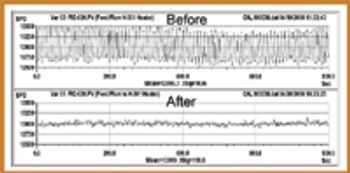
Innovations in process analysis and control offer significant opportunities for improving pharmaceutical manufacturing operations
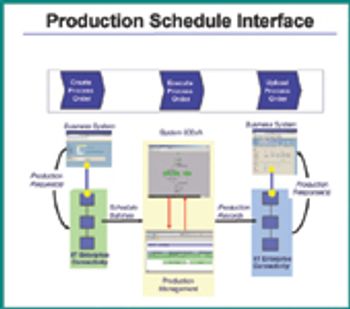
How can manufacturers improve efficiency and increase production while maintaining regulatory compliance? Process-automation solutions are one method.

New! QUMAS Introduces R&D Suite Complete with Integration and Services

The role of micro-biological testing in real-time release is too important to ignore.

It's what's on the outside that counts, too.

Brussels, Belgium (Jan. 11)-EPCglobal, the not-for-profit organization dedicated to driving global adoption of the Electronic Product Code (EPC), has ratified the electronic pedigree document specification.

Industry will be challenged to embrace new methods of supply chain collaboration.
In recent years, AI has become important in a number of fields in helping to make better use of information, increasing efficiency and enhancing productivity.

The US Food and Drug Administration has modified its requirements for drug pedigrees accompanying wholesale pharmaceutical transactions, following a US District Court preliminary injunction barring the agency from enforcing certain provisions of the rule that was to have gone into effect on Dec. 1.

Dublin, OH (Nov. 14)-A half-year Cardinal Health study of radio-frequency identification (RFID) tags "under real-world conditions has demonstrated that the technology has real promise to provide an added layer of safety," according Renard Jackson, the company's vice-president and general manager of global packaging services, in a prepared statement.

San Antonio, TX (Nov. 1)-Though the US Food and Drug Administration's final guidance on process analytical technology (PAT) was published in Sept. 2004, companies are still unsure about how exactly to implement PAT in their processes.
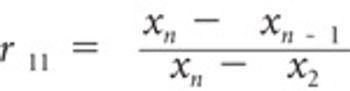
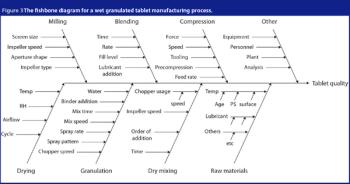
Outliers may provide useful information about the development and manufacturing process. Analysts use various statistical methods to evaluate outliers and to reduce their impact on the analysis. This article describes some of the more commonly used identification methods.
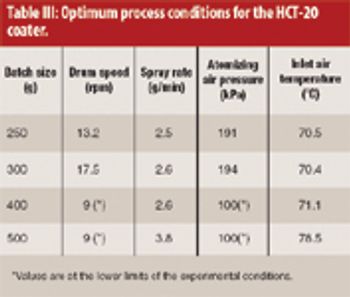
Large amounts of data are currently being generated in an attempt to understand and improve formulation, process, and manufacturing efficiency. This task requires novel data mining software systems tailored specifically for the formulator and process engineer.
This article examines what other industries have done to address the challenges associated with successfully implementing the principles and concepts of process analytical technology, particularly the specific elements of measurement, analysis, and control. The author explores how other industries' approaches can be applied directly to pharmaceutical manufacturing.
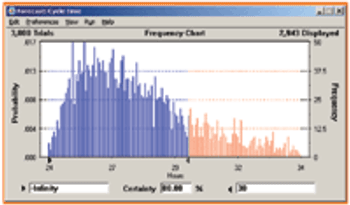
This article discusses the role of process simulation and scheduling tools in the development and manufacture of pharmaceutical products.
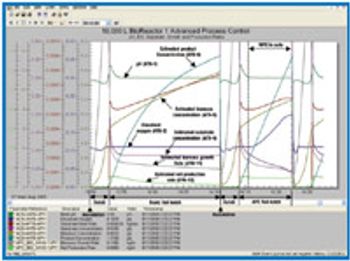
Modeling conducted in the process development and early commercialization stages can increase process efficiency and lead to process-control improvement. A virtual plant environment, running on a PC, can incorporate the same industrial control systems and configuration expertise used in manufacturing and helps many parts of process analytical technology. It also can make possible the enlightened use of technologies such as model predictive control, first-principal models, neural networks, and multivariate statistical process control.

CPhI Worldwide, Paris (Oct. 3)-Archimica, participating in its first CPhI Worldwide exhibition since being launched as new company earlier this year, outlined manufacturing expansions, technology additions, and recent projects at its facilities in the United States and Europe.

CPhI Worldwide, Paris (Oct. 3)-Exhibitors at CPhI Worldwide reported expansions, enhancements in technology, and new projects last week in Paris.

Arlington, VA (Sept. 12)-At the American Association of Pharmaceutical Scientists meeting here, "e;Real World Applications of PAT and QbD in Drug Process Development and Approval" (Sept. 11-12), chemical engineer and process modeler Michael L. Thompson, PhD, described how Procter & Gamble (West Chester, OH, www.pg.com) applies these mathematical tools to increase product quality and reduce development and trouble-shooting time for consumer and pharmaceutical products.

Washington, DC (Sept. 12)-The Office of New Drug Quality Assessment (ONDQA) in the Center for Drug Evaluation and Research (CDER) has approved one new drug application (NDA) under its CMC Pilot Program and has two more applications are under review. The pilot was established last year to provide an opportunity for FDA and industry to explore strategies for including Quality by Design (QbD) principles and process analytical technology approaches in regulatory submissions, explained ONDQA deputy director Chi-wan Chen at the PDA-FDA Joint Regulatory Conference here

A hybrid system using paper and electronic pedigrees will be needed.

Implementing an electronic system to track out-of-specification results could help ensure compliance with current good manufacturing practices, but the system must be 21 CFR Part 11 compliant and easy to install, maintain, and use.
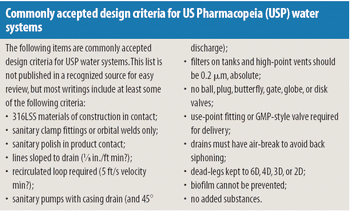
Water systems require a commonsense approach and good engineering rather than unquestioned acceptance.
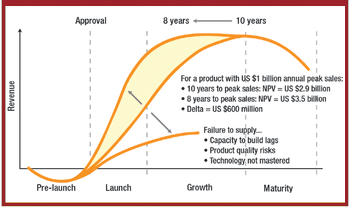
Companies must create a risk-based framework for developing and manufacturing drugs, and acquire the scientific knowledge and technological skills to create more complex products.