Information technology is the glue that should unify a company while ironically, it enables further fragmentation. Experts talk about the successes and challenges for IT in helping a company function efficiently.

Information technology is the glue that should unify a company while ironically, it enables further fragmentation. Experts talk about the successes and challenges for IT in helping a company function efficiently.

The senior director of Oracle's Life Sciences Business Unit tackles some of the technical issues regarding regulatory standardization, software integration, and the trend toward virtualization, among other things. Do you have something to ask Arvindh Balakrishnan? Click here to submit your questions.
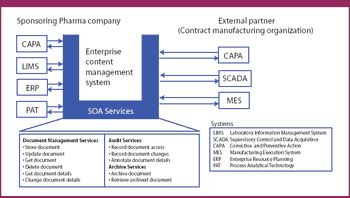
More than ever, drug makers need to be fleet and flexible-and they need their software to be the same.

Company Notes: Gibraltar expands facilities; Allozyne appoints new president and CEO, more.

Before any information technology solution can be installed, a company must decide whether applications are going to reside on individual computers at each employee's workstation, on servers within or outside of an organization, or on a vendor's website.

Seven Reasons Why Pharmaceutical Makers Are Adopting Mobile Technology
The scope and complexity of GRC requirements are expanding so rapidly that businesses are struggling to fulfill them despite an increased willingness on industry's part to apply additional GRC resources.

Company and People Notes: ImClone, BMS, and Merck form agreement; CRI Worldwide names new CEO, more.

Company and People Notes: Orexo acquires Biolipox, Avalon cofounder resigns, more...

Company and People Notes: Novartis and MIT to study continuous processing, GSK appoints Andrew Witty as CEO, more.

Company and People Notes: Thermo Fisher Scientific buys Priority Solutions International, Wyeth elects new president and CEO, more.

Carl Zeiss MicroImaging (Thornwood, NY) recently introduced its "Colibri" light-emitting diode (LED) light source for fluorescence microscopy. The product is "the only LED light source optimized for white-field microscopy systems," says Becky Homan, product manager for biomedical light microscopy.
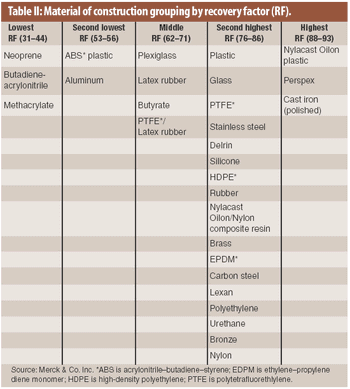
The material of construction is a factor in the recovery of residue in cleaning validation. An analysis of existing recovery data showed that recovery factors for drug products on various materials of construction may be categorized into several groupings.
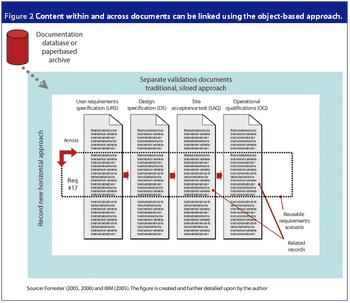
An electronic object-based approach towards validation is forecasted to be the upcoming validation paradigm supported by reliable web-based technology, organizational focus on risk management and overall enterprise effectiveness.

Company and People Notes: Evotec and Renovis enter agreement, Amgen to lay off 675 workers, more.

Company and People Notes: Catalent expands Bolton, UK, warehouse; Biotica appoints Edward E. Hodgkin as CEO and director; more.

Company and People Notes: Baxter and Halozyme Expand Relationship, Crucell Names COO, More.
In biomanufacturing, multiple sensors provide a wealth of data that could be used to enhance process understanding and assist in performance improvement. This article looks at how to move from a data-rich environment to one where the data are translated to useful information that leads to knowledge and, ultimately, process improvements.
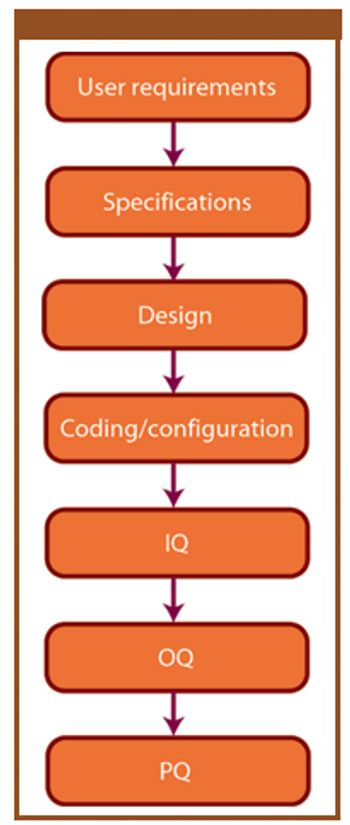
This article provides a historical review of computer validation in the pharmaceutical industry within the last three decades, evolving from the early years' initial concept and approach to today's current practices. Also included is how the regulations and industry have progressed in addressing the topic of computer validation.

Here's a look at some of the most interesting and thematic responses you-our readers-provided for Pharmaceutical Technology's anniversary survey of industry advances and directions.

Vendors who insist on using older and outdated technologies will eventually suffer increased pressure to follow the lead of those who adopt new technologies.

Brussels (May 30)-The European Federation of Pharmaceutical Industries and Associations (EFPIA) called for the pan-European and industry-wide adoption of 2-D barcode technology to combat the increase in counterfeit drugs in Europe.

Exhibitors' products prevent counterfeiting, provide child resistance, protect product quality, and improve packaging-line efficiency.

Signature authentication technology has evolved to become a stong tool for improving confidence in verification.

Dublin, OH (May 3)-In preparation for California?s new pedigree legislation, Cardinal Health plans to integrate radio frequency identification (RFID) technology into the operations at its pharmaceutical distribution center in Sacramento by fall 2007.