
Demand for custom drug delivery solutions is increasing and bringing forth an exciting period of valuable, innovative development opportunities.

Demand for custom drug delivery solutions is increasing and bringing forth an exciting period of valuable, innovative development opportunities.

The COVID-19 pandemic has created a rise in demand for R&D and a shift in focus for some contract organizations.

FDA approved hydroxychloroquine sulfate for emergency use as a treatment for some hospitalized patients with COVID-19.

Will moving at “warp speed” to develop a vaccine impact efficacy or safety?

Catalent and Johnson & Johnson announce joint investment and tech transfer to prepare for rapid scale-up and segregated cGMP commercial manufacturing capacity.

The new molecules entering the development pipeline are bringing forth exciting challenges in drug delivery.

Assays can provide a useful tool in determining the potential toxicity of drugs throughout the development cycle.

Can investing in early formulation studies drive a new therapy successfully across the commercialization finish line?

Under the agreement, ERS Genomics will license its gene-editing technology to Aelian Biotechnoloy to support its commercial functional genomic screening platform.

Contract partners must help innovators, especially smaller and virtual companies, consider manufacturability as early as possible in development. This requires focusing on technical and operational performance, as well as cost.

Catalent builds on its investment in cell and gene therapy development and manufacturing with the acquisition of MaSTherCell Global.
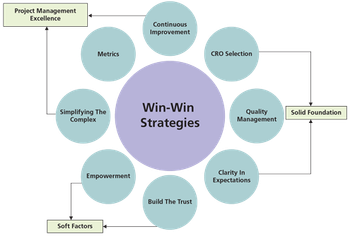
How to adopt win-win strategies and understand quality agreements for complying with cGMP when building strategic relationships with pharmaceutical contract research organizations.

CMOs and CDMOs expanded their services and facilities in 2019 and early 2020.

The need for manufacturing speed inspires contract manufacturers to explore advanced processing technologies.

CDMOs and CMOs will continue to invest in biopharmaceutical services and facilities as the bio/pharmaceutical industry looks to biosimilars and personalized medicine.

Drug development contract services company PPD announces initial public offering.

The acquisition expands Charles River’s scientific capabilities in cell therapy development.

Cambrex reports that the acquisition by the investment group will facilitate ongoing growth.

The companies announced a commercial supply agreement following FDA’s accelerated approval.

New training facilities, laboratories, packaging, gene therapy manufacturing, and biologics manufacturing highlight Thermo Fisher Scientific expansions.

Lubrizol Life Science Health adopts new name and opens commercial manufacturing facility.

Lonza, through its Ibex Solutions, will now cover preclinical and clinical development and manufacturing for a significant portion of Genmab’s pipeline.
Limited guidance and numerous challenges create confusion about the scope and timing of stability testing for drugs in development.
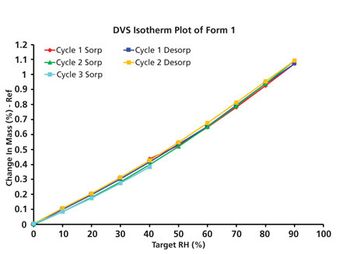
An understanding-during early development-of the solid form landscape of an API can enhance product quality and manufacturing processes.
New therapies, new technologies, global supply chain challenges, and political pressures draw pharma professionals to major industry event.