
Are strategic partnerships in clinical research a model for CMC services?

Are strategic partnerships in clinical research a model for CMC services?
Industry experts share their views on the outsourcing model and the current and future direction of contract chemical API manufacturing.
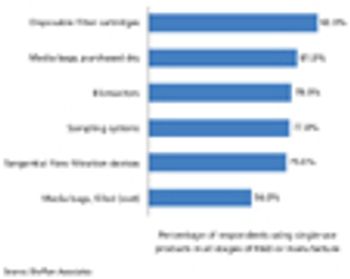
Advances in techniques and single-use systems are revolutionizing vaccine manufacturing.

Successful product development and technology transfer between a CMO and the sponsor company requires a multifaceted collaborative approach. The author, Marga Vines from Grifols International, analyzes a project for an injectable drug for which a new drug application was submitted to FDA.
External manufacturing plays a crucial role in pharmaceutical companies’ supply strategy. The author examines market trends for the captive and merchant global market for active pharmaceutical ingredients and intermediates.

Claudia Roth, President of Vetter Development Service USA, discusses trends in single-use technology for clinical manufacturing.

The US and EU move forward with measures to fortify the pharmaceutical supply chain.
As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

When speaking of partnerships, the discussion typically focuses on the relationship between a pharmaceutical/biopharmaceutical company as the sponsor company and a contract-service provider.

Patheon will acquire softgel-specialist company Banner Pharmacaps, based in North Carolina in the US, for $255 million

The author provides results and commentary on a survey analyzing outsourcing strategies, practices, challenges, and outcomes in the selection of a CRO.

The author discusses how a CDMO helps in gaining process understanding and in developing robust, high-quality products and processes.

Jim Miller, president of PharmSource, examines the future direction of CROs/CMOs and the factors influencing the pharmaceutical contract services sectors.

Sponsor companies' increasing focus on strategic outsourcing has changed the rules of the game.

A technical forum featuring Catalent Pharma Solutions, SAFC, and Neuland Laboratories.
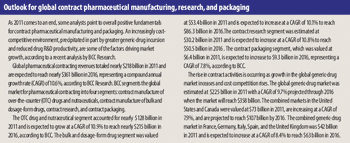
Expansion activity was limited as fine-chemical producers and CMOs of API and intermediates grapple with changing industry fundamentals.

Drug shortages, supply-chain security, generic-drug incursion, and flexible manufacturing models are some of the issues shaping the bio/pharma industry.

The global excipients market shows moderate growth, increased consolidation, and expansion activity in emerging markets and select product areas.

Biocatalysis, chemocatalysis, and other chiral technologies continue to attract the investment dollars of CMOs and fine-chemical companies.

The increased trend of outsourcing coupled with a relatively strong economy has seen the fine chemicals market grow at a very high level when compared to historical data.

As contract manufacturers and fine-chemical suppliers gather for CPhI/ICSE, effective strategies for technology differentiation are key in an increasingly competitive environment.

Avantor reviews its experience with the Rx-360 shared audit pilot program, which is aimed at protecting the pharmaceutical supply chain. This article is part of a special issue on Outsourcing.

A recent industry survey shows keen interest in improving bioreactors and cell-culture media.
Chemocatalytic and biocatalytic routes play an important role in improving the manufacture of intermediates and active pharmaceutical ingredients.