
Sponsor companies and contract development and manufacturing companies must ensure that the suppliers they use are following sustainability guidelines to truly produce ‘green’ products.

Sponsor companies and contract development and manufacturing companies must ensure that the suppliers they use are following sustainability guidelines to truly produce ‘green’ products.

Contractors are working with sponsor companies to establish and maintain sustainable manufacturing practices in pharma.

With its new offering, Lonza can tailor services to support smart capsule companies with its bi-layer capsule manufacturing technology.

Employing novel technologies and more patient-centric approaches can help to reduce the potential of formulation failure.

CordenPharma’s partnership with GENEPEP expedites lead development and validation in project phases, and its LNP β-sitosterol supports sustainable emission reduction targets.

The need for preclinical testing expertise is growing as molecular complexity increases.

A look at using best practice tech transfer methods for CGTs to increase process and analytics robustness while being scalable.

The new centralized hub will provide advanced testing of nucleic acids, which is expected to simplify mRNA substance testing.

The 65,000-square-foot facility is designed with the capacity and capability to help scale the next generation of CGTs for human trials and beyond.

Further expansions of the pharmaceutical development and manufacturing services of ten23 are happening at the BASE and VIVA facilities to continue to support complex sterile product development, testing, and manufacturing.

WuXi XDC will provide end-to-end services to support Boostimmune’s discovery of novel bioconjugates.

The facility is based on a state-of-the-art design concept that allows maximum flexibility for a multi-product, multi-platform offering.

The new portfolio from the acquisition will include contract research organization solutions, contract development and manufacturing organization solutions, and KSM/API solutions.
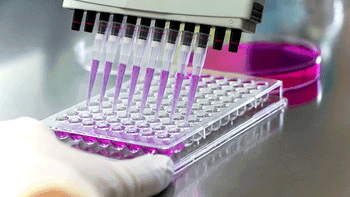
Contract testing services demonstrate a major increase in the speed and efficiency of testing using new testing methods and technologies.

Quotient Sciences’ facility expansion is designed to increase support for fully integrated drug development programs.

Shifting toward more technological solutions and ensuring a greater understanding of the workforce’s needs will give both CROs and sponsors a market advantage.

In the coming year, pharmaceutical innovation will be fueled by key trends throughout the quality sector.

The COVID-19 pandemic has led to many changes and shifts in the pharmaceutical industry, specifically with how pharmaceutical companies develop vaccines.

The Vault Quality Suite will help Adare simplify its quality operations across its sites in the United States, France, and Italy.

Public relations agencies dissect pharmaceutical trends: Brandwidth Solutions, Orientation Marketing and White Matter Communications

In this episode of the Drug Solutions Podcast, Meg Rivers discusses outsourcing strategies in biopharma with Jeff Henderson, key account manager of Vetter.

In this episode of the Drug Solutions Podcast, Chris Spivey interviews executives at Shabas Solutions LLC, who ran the overseas QMM pilot project.

The CPHI Annual Report 2022 predicts cash reserves from venture capitalists and private equities will drive expansion in the demand for contract research organizations and contract development and research organizations.

Under two new agreements, Societal CDMO will offer analytical, technical transfer, formulation, manufacturing, and packaging services for novel therapeutics.

Sponsors should explore key considerations ahead of choosing a new outsourcing partner.